Why "Zn" creates complicated compound, Wheather it is not a transition element?
1 Answer
Mar 28, 2018
As long as it has
The molecular orbitals formed in crystal field theory don't tell the whole story. Ligand field theory includes the interactions that form the
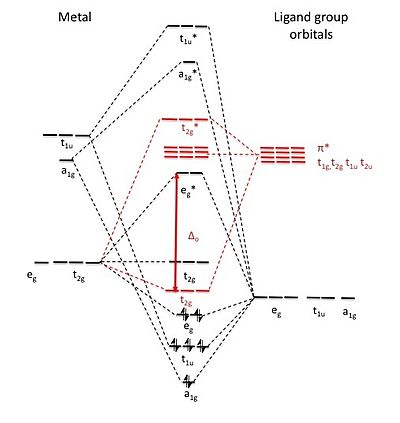
Such an MO diagram would belong to a
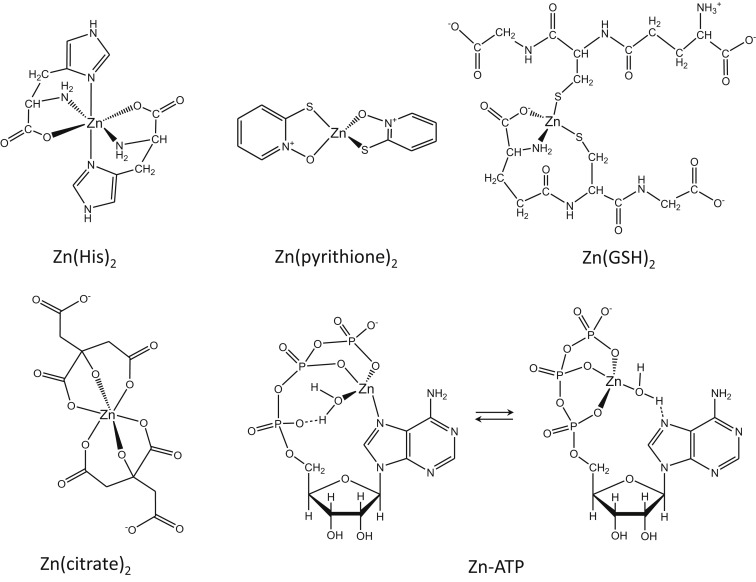
So, there is no issue with whether a ligand can donate electrons or not to bond with

