Why is rhenium frequently used as a catalyst? What properties does it have that lend to it being a good catalyst?
1 Answer
Well, to be clear, we often really mean rhenium-containing catalysts, but sometimes rhenium metal itself could work. This article goes into much detail on rhenium.
Some nice properties about rhenium metal (some from the article):
-
#"Re"# metal itself is relatively inert with respect to water and nonoxidizing acids (such as#"HCl"# ). -
Its most stable oxidation state is actually
#bb(+7)# , whereas the most stable oxidation state of#"Mn"# is#+2# (although it could reach#+7# ). This is partially why it can take on a lot of ligands to make complexes with high coordination numbers. -
#"Re"# is also the biggest stable metal in its group (ignoring the synthetic element#"Bh"# , bohrium), and thus it uses big#d# and#s# (and#f# ) orbitals to bond.
Some interesting info about rhenium-based catalysts:
- Rhenium-based catalysts are typically highly-selective, namely the rhenium oxides, sulfides, selenides, or even the metal itself. (Of course, this is not always the case. Rhenium carbonyl halides, for example, need higher temperatures to work, and decompose into the metal.)
This is particularly true for hydrogenation of organic compounds, where
#"C"="C"# (olefinic bonds),#"C"="O"# , nitro, and aromatic groups are targeted, and often#"C"-"X"# and#"C"-"S"# bonds are not (where#X# is a halogen).Here are a few examples:
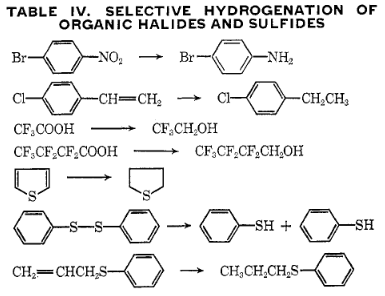
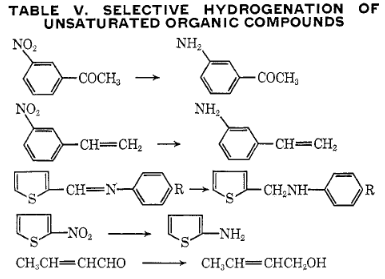
- It has been found that a small addition of
#"Re"# metal into nickel-based catalysts typically increases their activity and stability. We call this a "promoter effect".
The article mentions, for example, that cottonseed oil is hydrogenated
#50 - 70%# faster with only#0.1 - 0.3%# rhenium mixed in with Raney nickel.More promoter effects can be seen when adding trace
#"Re"# into#"Cu"# ,#"Co"# ,#"Mo"# , or#"V"# -based catalysts as well, when it comes to oxidizing olefins and aromatics.
Anyways, there are plenty more applications of rhenium-based catalysts that are discussed here.

