Why does #"NaOH"# only react with one acidic proton in salicylic acid even though both the carboxyl and the hydroxyl groups could potentially react with it?
↳Redirected from
"What covalent compounds are soluble in water?"
#"NaOH"# would react as a base with only one of these protons on salicylic acid for the first deprotonation:
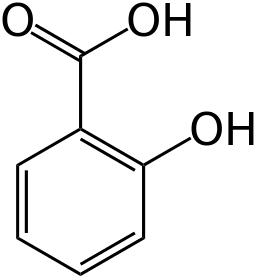
Yes, technically, salicylic acid has two acidic protons. But there is a significant difference in their acidities. Each proton has its own #"pKa"#, which is a way of measuring acid strength. More negative / less positive #"pKa"# means the proton is more acidic (its #"O"-"H"# bond is weaker) and it is easier for it to get deprotonated by #"OH"^(-)# first.
This is a thermodynamic quality, and is therefore also a measure of the energetic favorability of one proton's dissociation over another for the first deprotonation.
- Carboxylic acid protons in general usually have a #"pKa"# around #5#.
- Alcoholic protons in general usually have a #"pKa"# around #15 - 17#.
Remember that a difference of #1# on a #"p"# scale (such as #"pH"#, #"pKa"#, etc) is an order of magnitude. For example, a #"pKa"# of #4# vs. #5# means that the compound with #"pKa"# #5# is #10# times more basic.
So, it is over #10000000000# (#10^10#) times more energetically favorable for the carboxyl proton to come off first by reaction with #OH^(-)# than for the alcoholic proton to come off first.


