why [CuCl4]2- has a flattened tetrahedral structure, not regular tetrahedron and [CoCl4]2- is tetrahedron?
1 Answer
It's probably a Jahn-Teller distortion.
From the electron configuration of the atoms:
#"Cu": [Ar] 3d^10 4s^1#
#"Co": [Ar] 3d^7 4s^2#
Take away two electrons and you have the
#"Cu"^(2+): [Ar] 3d^9 4s^0#
#"Co"^(2+): [Ar] 3d^7 4s^0#
Therefore, we have a
Their ORIGINAL tetrahedral d-orbital splitting diagrams would look like:
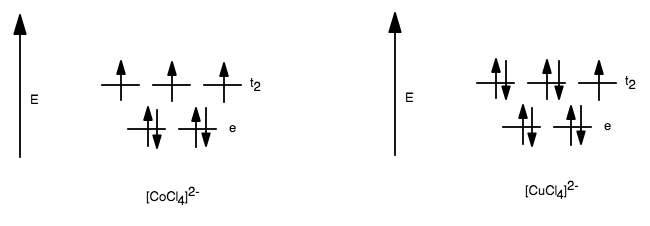
But we can see that there are multiple ways to fill the orbitals in
Three ways, actually, because the
#ul(uarr darr)" "ul(uarr darr)" "ul(uarr color(white)(darr))# #" "bb((1))#
#ul(uarr darr)" "ul(uarr color(white)(darr))" "ul(uarr darr)# #" "bb((2))#
#ul(uarr color(white)(darr))" "ul(uarr darr)" "ul(uarr darr)# #" "bb((3))#
Therefore, to relieve this degeneracy (at least somewhat), a Jahn-Teller distortion occurs to descend in symmetry from tetrahedral
[This is analogous to the Jahn-Teller distortion that forces the octahedral
#O_h# symmetry to descend to square bipyramidal#D_(4h)# .]
Here is how each irreducible representation correlates:
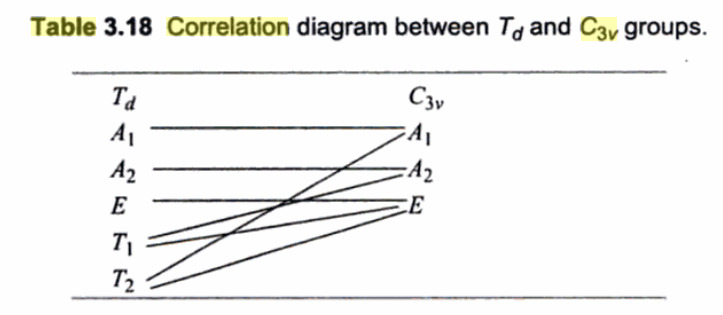
We have:
#E -> E#
#T_2 -> A_1 + E#
The orbitals will therefore split in energy as follows:
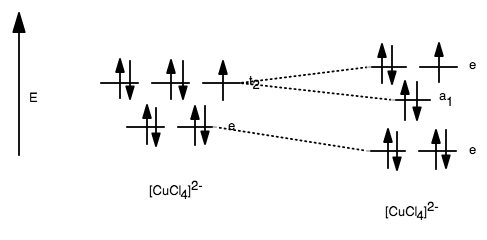
As it turns out, this is not going to completely lift the degeneracy. There is still a double degeneracy in the
The transition metal complex distorts as follows:
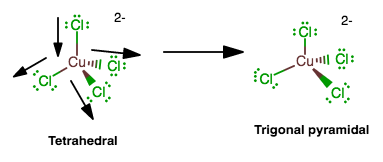
and if the bonds stretch in the other direction, the orbitals will be in order of energy as
#e, e, a_1# instead of#e,a_1,e# .

