Why are the convergence lines in the UV spectrum used to calculate the ionization energy of hydrogen rather than the convergence line in the visible spectrum?
The lines in the UV emission spectrum of hydrogen gas converge at 9.12 x 10^-8
The lines in the UV emission spectrum of hydrogen gas converge at 9.12 x 10^-8
1 Answer
For the same reason you shouldn't look in the infrared for convergence lines... the ionization energy is by definition using the initial state as the state the atom exists at room temperature, i.e. the ground state.
In general, you should be using the convergence lines that you get for the transitions because the energy levels go as
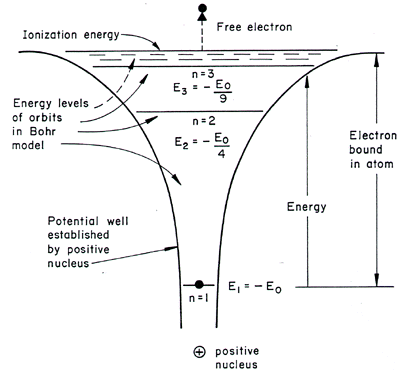
The atom is in the ground state at room temperature, 99% of the time.
You shouldn't see a convergence in the visible region for the Lyman series transitions, which start in the ground state as hydrogen atom normally does at room temperature, because this series is in the UV part of the EM spectrum, and not the visible.

If you look in the visible, which is higher wavelength (lower energy), you may be looking at higher-order transitions (specifically, Balmer) that involve excited final energy states, which are much less populated at room temperature.
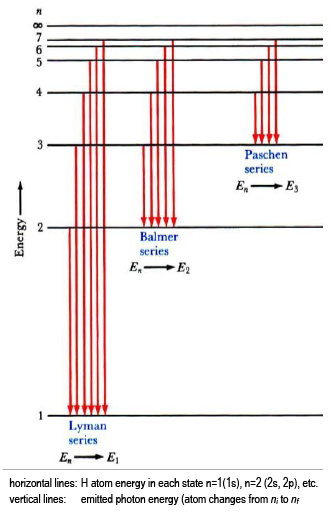
Given the appropriate amount of light, you can see a stark difference in the intensities of the series past the Lyman series, which makes them much less useful to observe.

