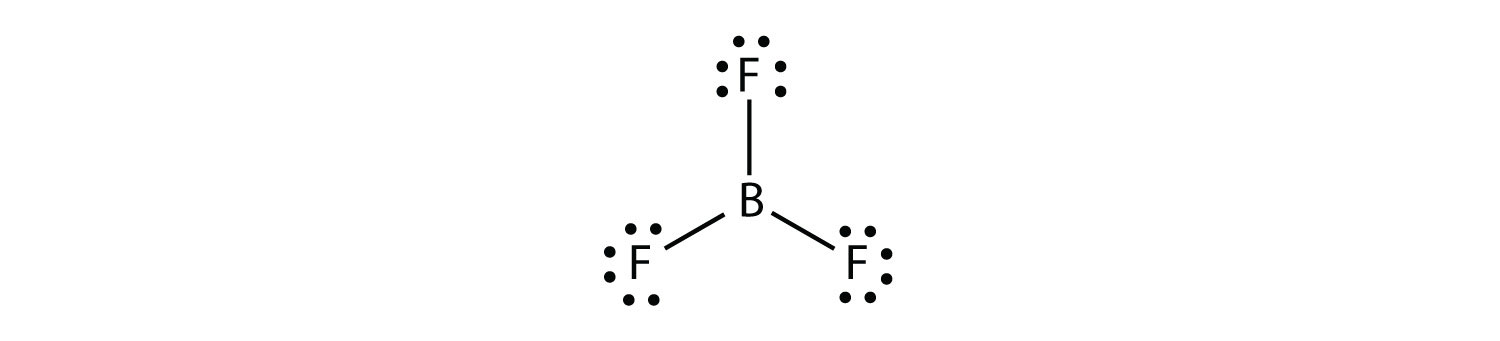
Which one of the following Lewis structures is definitely incorrect?
Which one of the following Lewis structures is definitely incorrect?

Which one of the following Lewis structures is definitely incorrect?

1 Answer
None of the above! These are all right!
I'm not sure who wrote this question, but they are all real molecules and correct structures... Here they are from a google search...
#"Xe"# comes in with#8# valence electrons.- Each
#"O"# comes in with#6# valence electrons.
That gives
That gives (again, lone pairs not seen on the




