Which of the following complexes exhibit the highest paragmagnetic behavior? a. [Co(ox)2(OH)2]^- b. [Ti(NH3)6]^3+ c. [V(gly)2(OH)2(NH3)2]^+ d. [Fe(en)(bpy)(NH3)2]^2+
1 Answer
I found
I would first reference your text to check the charge of the ligands, but you would have to memorize these in an exam. I have a naming guide here.
#"ox"# is oxalato, a#-2# bidentate ligand.#"gly"# is glycine, a neutral ligand at#"pH"# #7# . Since it has a carboxyl group, it is possible that it binds#eta^2# . It is also possible that it binds bidentate at basic#"pH"# .#"OH"# is hydroxide, a#-1# monodentate ligand.#"en"# and#"bipy"# are both neutral bidentate ligands.
From that, we can then determine the oxidation states on each metal and thus their number of unpaired electrons.
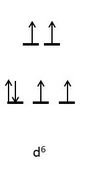
#["Co"("ox")_2("OH")_2]^(-)# has two#-2# bidentate and two#-1# monodentate ligands, giving a#+3# oxidation state on cobalt, making it a#d^6# metal in the octahedral ligand field.
Since
#"ox"^(2-)# and#"OH"^(-)# are both fairly weak-field ligands (weaker sigma donors than water), this is likely a high spin#d^6# complex, with four unpaired electrons.
-
#["Ti"("NH"_3)_6]^(3+)# has three neutral ammine ligands, each strong-field, making this a low-spin complex involving a#d^1# metal. Only one unpaired electron. -
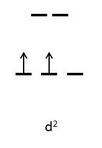
#["V"("gly")_2("OH")_2("NH"_3)_2]^(+)# has two#-1# hydroxo ligands, two neutral ammine ligands, and two neutral monodentate#eta^2# glycine ligands (at#"pH"# #7# ), making this#"V"^(+3)# , a#d^2# metal with two unpaired electrons in the octahedral ligand field splitting diagram.
I did not need to think about strong- or weak-field for either of the previous two complexes. Why is that?
#["Fe"("en")("bpy")("NH"_3)_2]^(2+)# has two neutral bidentate ligands (ethylenediamine and 2,2'-bipyridine), and two neutral monodentate ammine ligands, making iron a#+2# oxidation state. We therefore have a#d^6# metal in an octahedral ligand field consisting of three species of strong-field ligands, giving a low-spin#d^6# configuration, having diamagnetism.


