What is the electron count for these complexes??
1) Cp2Ta(CH2)2 Ir(CO)-(PPh3)
2) Ph2P(CH2)2 Ir(CO)-(PPh3)
1) Cp2Ta(CH2)2 Ir(CO)-(PPh3)
2) Ph2P(CH2)2 Ir(CO)-(PPh3)
1 Answer
Starred are the central atoms.
#"Cp"_2stackrel("*")"Ta"("CH"_2)_2stackrel("*")"Ir"("CO")("PPh"_3)# :
#14e^(-) + 16e^(-)# (respectively for each metal center)
#"Ph"_2stackrel("*")"P"("CH"_2)_2stackrel("*")"Ir"("CO")("PPh"_3)# :
#8e^(-) + 16e^(-)# (respectively for each central atom)
Drawing out the first molecule, I get:
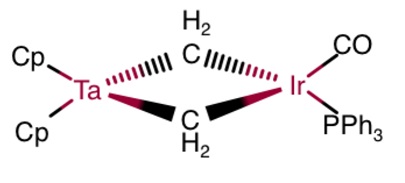
The second molecule is isostructural with the first.
DONOR-PAIR METHOD
-
Using the Donor-Pair method, I would assign two electrons per
#"CH"_2# bond, treating the#"CH"_2# carbenes as#-2# ligands that donate a total of#4# valence electrons, i.e.#""^((-2)):ddot"C""H"_2# . A#-1# charge is then given to each half of the complex per#"CH"_2# . -
Tantalum often forms the
#+5# oxidation state, and iridium is fine with a#+1# oxidation state. #"Cp"# is#"C"_5"H"_5# , which as a 5-electron donor is#"C"_5"H"_5^(-)# , cyclopentadienyl anion.#"CO"# and#"PPh"_3# are both 2-electron#sigma# donors and#pi# acceptors.
From this, around the tantalum center we have:
#"Ta"^(+5):# #0e^(-)# (noble gas configuration)
#2 xx "Cp":# #2xx5e^(-)#
#ul(2 xx "CH"_2: 2xx2e^(-))# (2 per bond)
#"Total" = 14e^(-)#
The total charge adds up around the tantalum center as
From this, around the iridium center we have:
#"Ir"^(+1):# #8e^(-)# (one#6s# and seven#5d# electrons)
#"CO":# #2e^(-)#
#"PPh"_3: 2e^(-)#
#ul(2 xx "CH"_2: 2xx2e^(-))# (2 per bond)
#"Total" = 16e^(-)#
The total charge adds up around the iridium center as
NEUTRAL LIGAND METHOD
If you want to do a different electron-counting method (the Neutral Ligand Method)...
If
Around
The result is then:
#"Ta"^(+1):# #4e^(-)# (noble gas configuration)
#2 xx "Cp":# #2xx4e^(-)#
#ul(2 xx "CH"_2: 2xx1e^(-))# (1 per bond)
#"Total" = 14e^(-)#
#"Ir"^(-1):# #10e^(-)# (one#6s# and seven#5d# electrons)
#"CO":# #2e^(-)#
#"PPh"_3: 2e^(-)#
#ul(2 xx "CH"_2: 2xx1e^(-))# (2 per bond)
#"Total" = 16e^(-)#
And that's good, we got the same result in the end. It should not matter either way.
I'll leave it to you to do
You should get

