What are acidic H atoms in these molecules?
b) CH3CH2CH(CH3)-C=O-CH3
c) HOCH2-CH2-C=O-C#-=# C-CH3
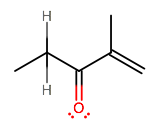
d) CH3 CH2 C=O C(CH3)=CH2
e) 1-COOCH3-2-CH2Cn benzene
f) COCl-cyclopentyl

b) CH3CH2CH(CH3)-C=O-CH3
c) HOCH2-CH2-C=O-C
d) CH3 CH2 C=O C(CH3)=CH2
e) 1-COOCH3-2-CH2Cn benzene
f) COCl-cyclopentyl
1 Answer
Well, I can only go off of the molecules I can understand from what you wrote, which are
Acidic
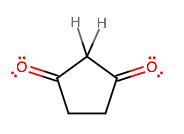
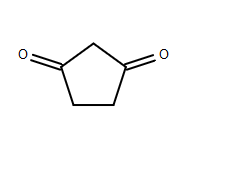
As mentioned, the acidic protons are on the
This is because the carbonyl oxygen(s) are electron-dense electron-withdrawing groups, thus making the
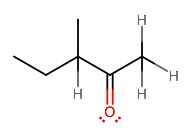
You can tell you have two choices here. Which proton is more acidic, left (more substituted) or right (not substituted)?
My bet is on the right-hand one, since two alkyl groups are donating electron density into carbon-3 (methyl and ethyl), which promotes the ability of carbon-3 to share electron density with the proton and makes the left-hand proton less acidic.
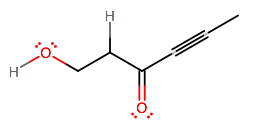
Well, it's either the
#alpha# protons on acetone have a#"pKa"# around#20# - on regular alcohols (e.g. ethanol, methanol, propanol, . . . ), it's typically around
#15 - 17# .
We expect further that the carbonyl oxygen withdraws electron density away from the hydroxyl oxygen, weakening the bond with its proton even more.
So, it is likely the hydroxyl proton here that is most acidic. A similar compound here has a comparable
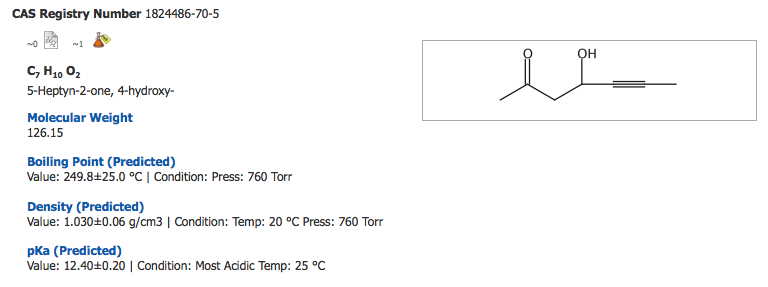
At this point, this one should be pretty obvious. There are no
This one should now be similarly obvious; it's the only
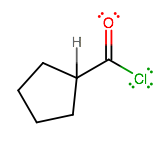
Try drawing the enolate resonance structure. Where would the added electrophile attach?

