#sp^3# hybridization - can somebody explain what my book is trying to say?
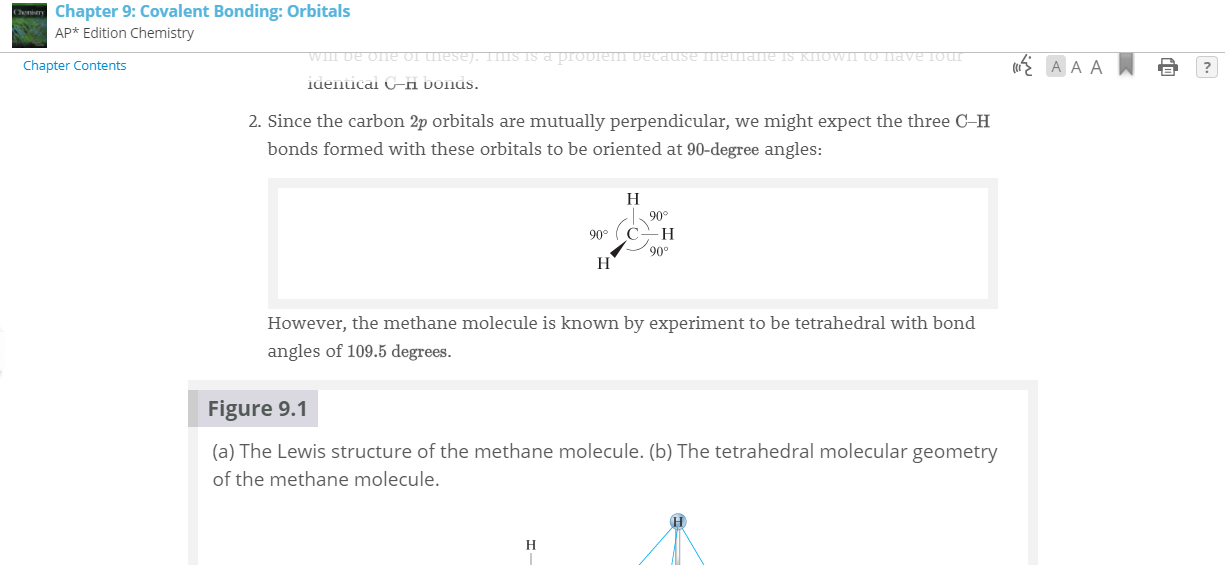
This is for #CH_4# . I understand that the electron configuration for #C# contradicts what actually happens in bonding, but I don't understand what #2 is trying to say.

This is for
1 Answer
All it is saying is that invoking hybridization, i.e. mixing of pure, differing atomic orbitals to form new, identical orbitals that are not rigidly
Recall that the four valence atomic orbitals (i.e. pure orbitals) of carbon are the
The
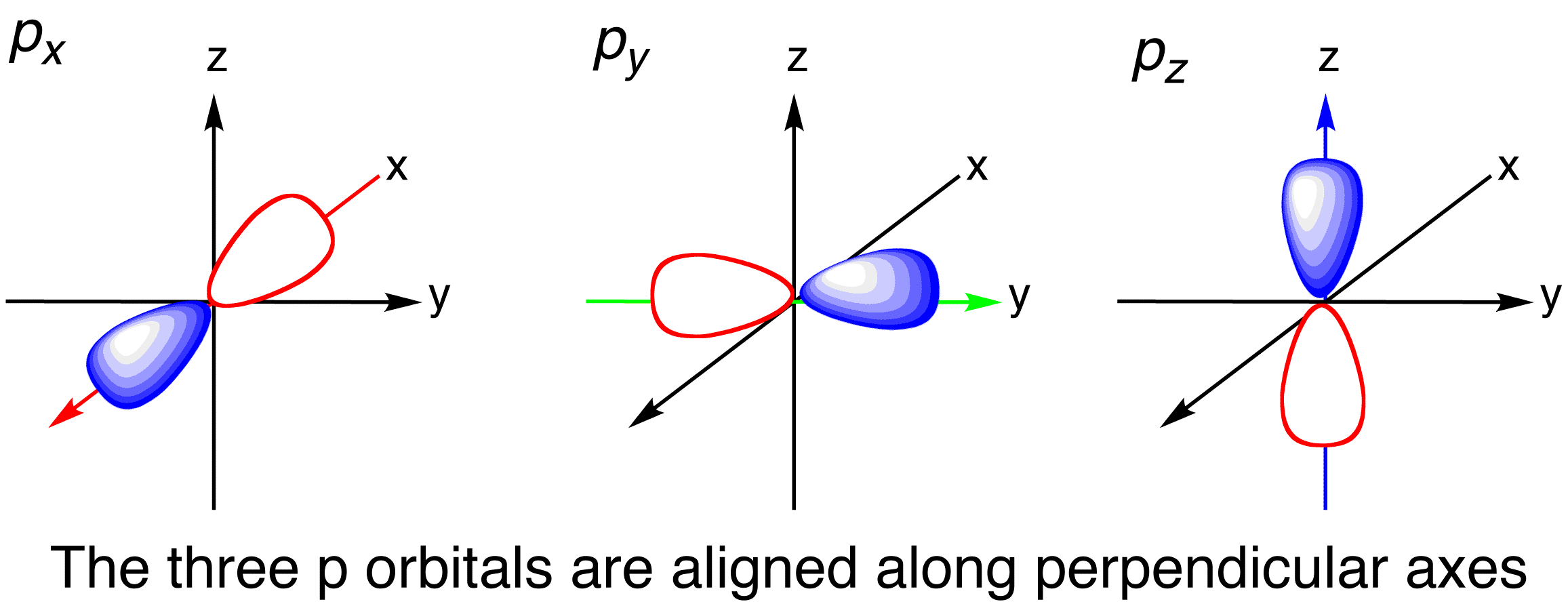
Since the
We did not observe
but we did observe
The pure

