PLEASE Help! with Reactants decreasing in Molarity?
Rate constant = 0.80 M^-1 s^-1 at 300℃
2nd order Reaction
Calculate the time it takes for the Initial reactant concentration to decrease from 0.072M to 0.054M
Please Help by showing my how to tackle this equation in steps! Thank you!
Always,
Ozzy
Rate constant = 0.80 M^-1 s^-1 at 300℃
2nd order Reaction
Calculate the time it takes for the Initial reactant concentration to decrease from 0.072M to 0.054M
Please Help by showing my how to tackle this equation in steps! Thank you!
Always,
Ozzy
1 Answer
I got about
Set up the rate law.
#r(t) = k[A]^2#
as it would be for a second order reaction with one reactant. I assume you mean
#A -> B#
To solve this, you only have the rate constant
The initial rate of reaction ought to be near the start of the reaction, and you were given
#Delta[A] = "0.072 M" - "0.054 M"#
The tangent line that touches the start of reaction has to touch
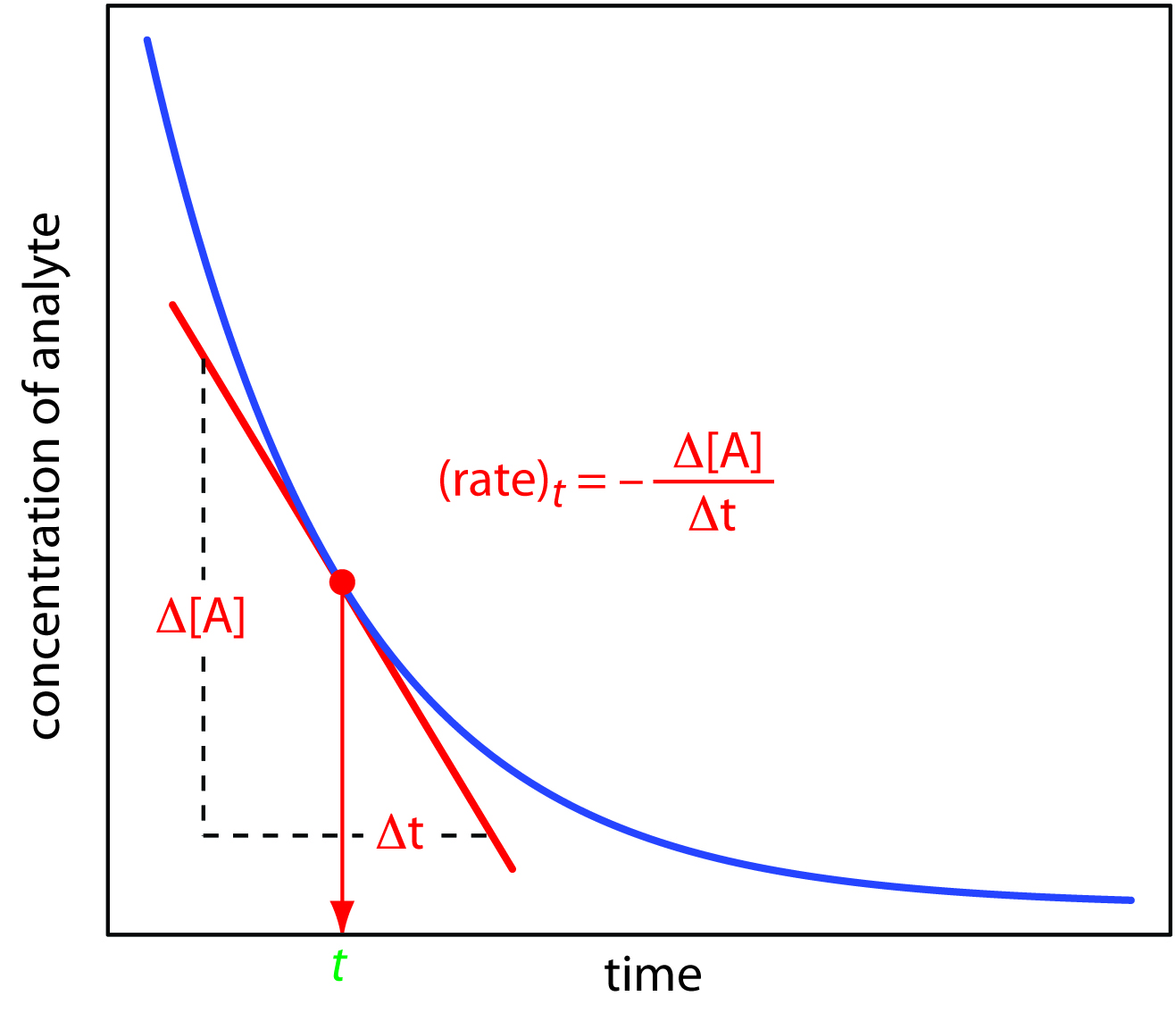
Assuming
#-(Delta[A])/(Deltat) ~~ r(t)#
We assume that the coefficient of
#r(t) ~~ "0.80 M"^(-1)cdot"s"^(-1)("0.072 M")^2#
#= 4.15 xx 10^(-3) "M/s"#
Now we can equate that to the change in concentration over time in this presumably short time interval.
#-(Delta[A])/(Deltat) ~~ 4.15 xx 10^(-3) "M/s" ~~ -("0.054 M" - "0.072 M")/(Deltat)#
Therefore:
#color(blue)(Deltat) ~~ -("0.054 M" - "0.072 M")/(4.15 xx 10^(-3) "M/s")#
#=# #color(blue)("4.34 s")#

