List the similarities and differences between ionic, covalent, and metallic bonds?
1 Answer
Here's the simplest way to view these:
#ul("Ionic"" "" "" "" ""Covalent"" "" "" "" ""Metallic")#
#"Metal+"" "" "" ""Nonmetal+"" "" "" ""Metal+"#
#"Nonmetal"" "color(white)(/.)"Nonmetal"" "" "" "color(white)(..)"Metal"#
Ionic bonds are in general bonds where each atom significantly differs in electronegativity, the affinity for electrons:
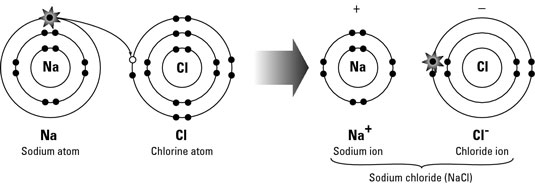
Common examples are
Covalent bonds are chemical bonds formed by sharing electrons between nonmetals:

Common examples are
Metallic bonds are formed by spreading electrons between metal cations in a metallic network:

It could be between the same metal, or different metals, i.e. alloys made from elements on the left-hand side of the periodic table and/or the
A common example is brass, which might have

