How to determine the temperature change for dissolving #"10.0 g"# of #"KClO"_4(s)# into water?
A practice problem in my textbook says...
"Potassium perchlorate has a lattice energy of -599 kJ/mol and a heat of hydration of -548 kJ/mol.
Find the heat of solution and determine the temperature change that happens when 10.0g of potassium perchlorate is dissolved with enough water to make 100.0 mL of solution.
(heat capacity of solution=4.05 J/g*C, density of solution= 1.05 g/mol."
I found the heat of solution as 51 kJ/mol, but I'm not sure where to go from here. I got 12.2 Celsius but that's not the answer. Thanks in advance!
A practice problem in my textbook says...
"Potassium perchlorate has a lattice energy of -599 kJ/mol and a heat of hydration of -548 kJ/mol.
Find the heat of solution and determine the temperature change that happens when 10.0g of potassium perchlorate is dissolved with enough water to make 100.0 mL of solution.
(heat capacity of solution=4.05 J/g*C, density of solution= 1.05 g/mol."
I found the heat of solution as 51 kJ/mol, but I'm not sure where to go from here. I got 12.2 Celsius but that's not the answer. Thanks in advance!
1 Answer
The solution cooled by
I would construct a thermodynamic cycle like this:
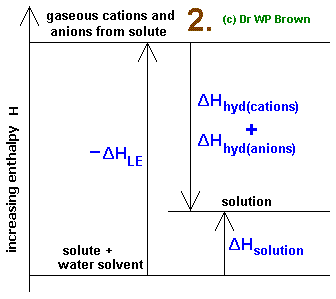
Here up is positive and down is negative, so:
#-DeltaH_L = +"599 kJ/mol"#
#DeltaH_"hyd" = -"548 kJ/mol"#
with
#"51 kJ/mol KClO"_4(s)#
to turn
When some amount of
Hence, we treat the solution as the surroundings and the ions as the system.
Given the solution density (not in
#100.0 cancel"mL soln" xx "1.05 g"/cancel"mL" = "105.0 g soln"#
Since
#"51 kJ"/(cancel("1 mol KClO"_4) xx "138.55 g KClO"_4/cancel"1 mol") = (q_"solute"" kJ")/("10.0 g KClO"_4)#
#q_"solute" = "3.681 kJ"# absorbed by the solute from the surrounding solution.
By conservation of energy,
#q_"solute" + q_"solvent" ~~ 0# ,
so
Given the specific heat capacity of the solution and knowing its mass from earlier, the heat involved in changing the temperature of the solution is:
#q_"solvent" ~~ m_"soln"C_(P,"solvent")DeltaT#
And so, we can plug in what we know to solve for the change in temperature:
#-"3681 J" = "105.0 g soln" xx "4.05 J/g"^@ "C" xx DeltaT#
#=> color(blue)ul(DeltaT = -8.66^@ "C")#
And so the solution cooled, as we would expect from an endothermic process (such as ice packs).

