How is the boiling point relate to vapor pressure?
1 Answer
An old question....and an old problem....
Explanation:
The boiling point of a liquid are those conditions of temperature and pressure, when the VAPOUR pressure of a liquid is equal to the ambient pressure, and bubbles of vapour form directly in the liquid. The
And this underlies the principle of vacuum distillation. A vacuum pump can SUBSTANTIALLY reduce the ambient pressure...and at an accessible temperature, the vapour pressure of the liquid may be
AS a first approx. we can sometimes use a vapour pressure nomograph to approximate the boiling point of a liquid AT REDUCED pressure...
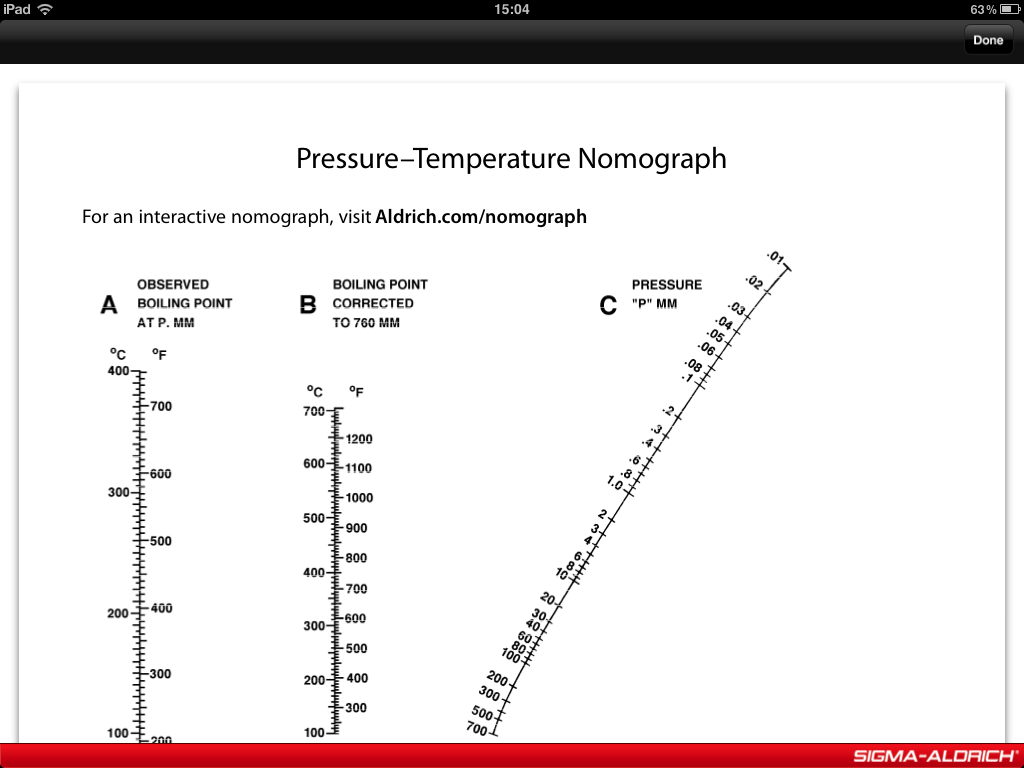
I know there is such a nomograph on the front of the

