How does alpha decay help stabilize a nucleus?
3 Answers
Alpha decay stabilizes a nucleus by moving it closer to the belt of stability.
During alpha decay, the nucleus ejects an alpha particle. The mass number of the nucleus decreases by 4 units and the number of protons decreases by 2.
Alpha decay is associated with heavy, unstable nuclei.
The stability of a nucleus depends on its neutron/proton ratio and on the number of nucleons. The graph below is a plot of the number of neutrons versus the number of protons in various stable isotopes.

The stable nuclei are in the blue band known as the belt of stability.
Alpha decay stabilizes nuclei that are above the belt of stability by lowering both the mass number and the atomic number. No stable isotopes exist above atomic number 83. These nuclei can come closer to the belt of stability by alpha decay.
Alpha decay stabilizes a nucleus by moving it closer to the belt of stability.
During alpha decay, the nucleus ejects an alpha particle. The mass number of the nucleus decreases by 4 units and the number of protons decreases by 2.
Alpha decay is associated with heavy, unstable nuclei.
The stability of a nucleus depends on its neutron/proton ratio and on the number of nucleons. The graph below is a plot of the number of neutrons versus the number of protons in various stable isotopes.

The stable nuclei are in the blue band known as the belt of stability.
Alpha decay stabilizes nuclei that are above the belt of stability by lowering both the mass number and the atomic number. No stable isotopes exist above atomic number 83. These nuclei can come closer to the belt of stability by alpha decay.
Alpha decay stabilizes a nucleus by moving it closer to the belt of stability.
During alpha decay, the nucleus ejects an alpha particle. The mass number of the nucleus decreases by 4 units and the number of protons decreases by 2.
Alpha decay is associated with heavy, unstable nuclei.
The stability of a nucleus depends on its neutron/proton ratio and on the number of nucleons. The graph below is a plot of the number of neutrons versus the number of protons in various stable isotopes.
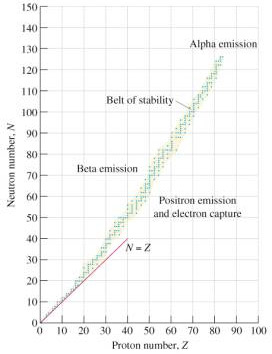
The stable nuclei are in the blue band known as the belt of stability.
Alpha decay stabilizes nuclei that are above the belt of stability by lowering both the mass number and the atomic number. No stable isotopes exist above atomic number 83. These nuclei can come closer to the belt of stability by alpha decay.


