How can we account for the color change of the solution?
Silver nitrate
Silver nitrate
1 Answer
Well, my best guess at what you're talking about is that maybe you're doing a Le Chatelier's Principle lab where you're reacting
#overbrace("Co"("H"_2"O")_6^(2+))^(color(pink)"pink") + 4"Cl"^(-)(aq) rightleftharpoons overbrace("CoCl"_4^(2-)(aq))^(color(blue)"blue") + 6"H"_2"O"(l)#
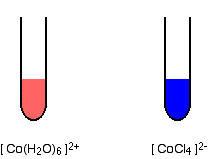
In lab, you may have been given
By adding
#"Cl"^(-)(aq) + "Ag"^(+)(aq) rightleftharpoons "AgCl"(s)#
to occur. This consumes pretty much all of the
As a result, Le Chatelier's principle suggests that the reaction shifts towards the reactants in the original reaction to restore
That's a pink, probably clearer, solution.
Students in lab typically see a change from translucent purple to cloudy pink on the bottom with a fluffy white layer on top.

