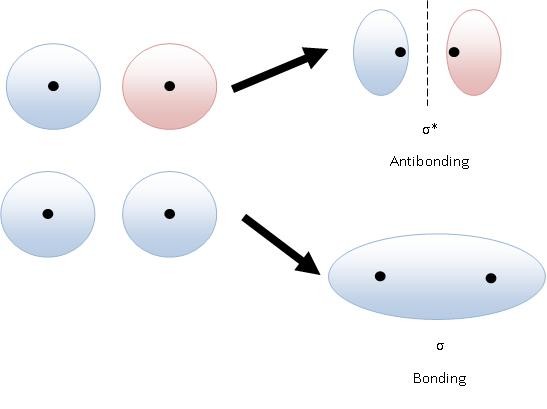
How are bonding and antibonding orbitals different?
↳Redirected from
"How many antibonding orbitals are there in Benzene?"
1 Answer
Jul 25, 2015
Clearly, bonding orbitals promote bonding. More specifically, they promote higher electron densities, meaning high orbital occupation probabilities. Antibonding orbitals promote electronic nodes or nodal planes, meaning low orbital occupation probabilities.
For example: