Explain how each of the following affects the vapour pressure of a liquid: (a) volume of the liquid (b) surface area (intermolecular forces) (c) temperature (d) increasing density?
1 Answer
Vapor pressure is an intensive property, since it only depends on the fraction of the surface particles that can vaporize.
The volume of the liquid might indirectly affect the vapor pressure... in the limit of small headspace.
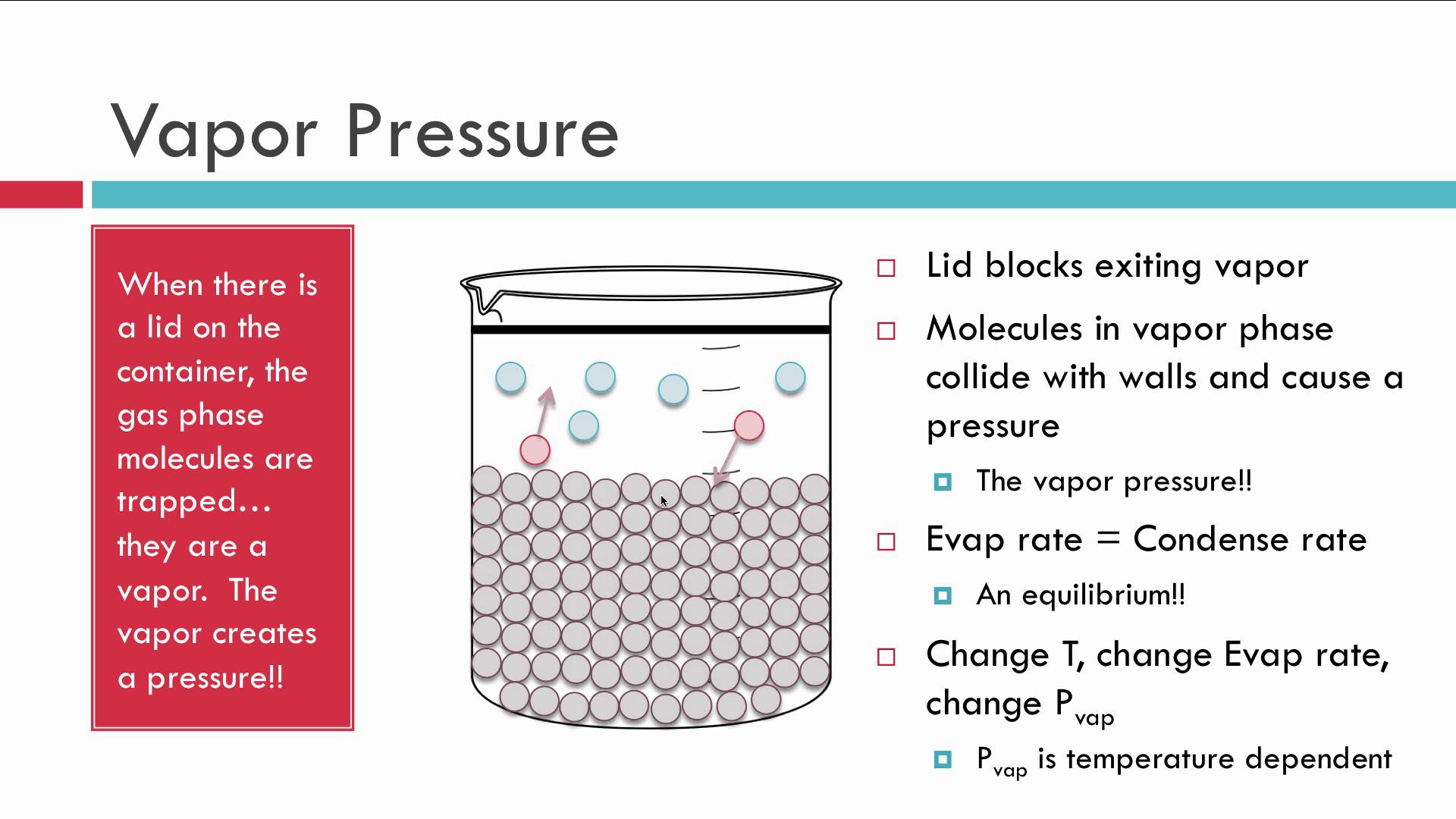
If the container is closed, and then filled with the liquid, the vapor pressure decreases as the container is filled.
The headspace volume consequently decreases, and since there is less room for gas particles to vaporize and exert a vapor pressure, the vapor pressure decreases until the container is full.
[NOTE: While the container is far from full, this has minimal effect on the vapor pressure.]
Surface area (an extensive property) has no influence on the vapor pressure, which is again, an intensive property.
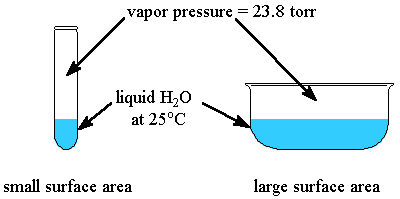
However, the intermolecular force strength at the surface would directly influence the surface tension, which varies inversely with the vapor pressure.
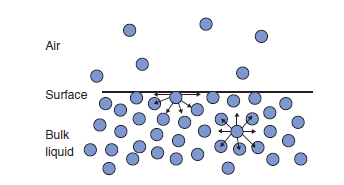
Higher intermolecular force strength, higher surface tension and lower vapor pressure.
Higher temperature increases the vapor pressure, as can be seen on most phase diagrams.
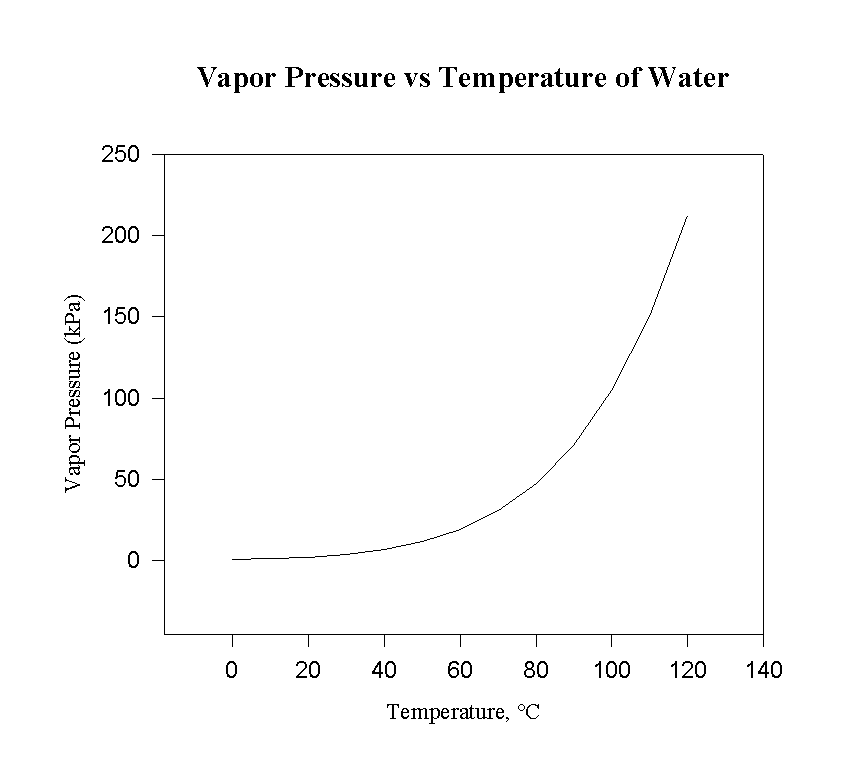
In most cases, the liquid-vapor coexistence curve has a positive slope, so a plot of
#ln P# vs.#1/T# returns a negative slope on a Clausius-Clapeyron plot.
Higher density decreases vapor pressure.
Think of it as an extreme application of external pressure...
The denser the condensed phase, the stronger the intermolecular forces that hold the substance together, so the less easily the surface particles can vaporize.
Hence, the vapor pressure above the liquid decreases for increasing liquid density.

