Can someone please explain me? I have some questions about the Periodic Table...(sorry for any grammar mistake)
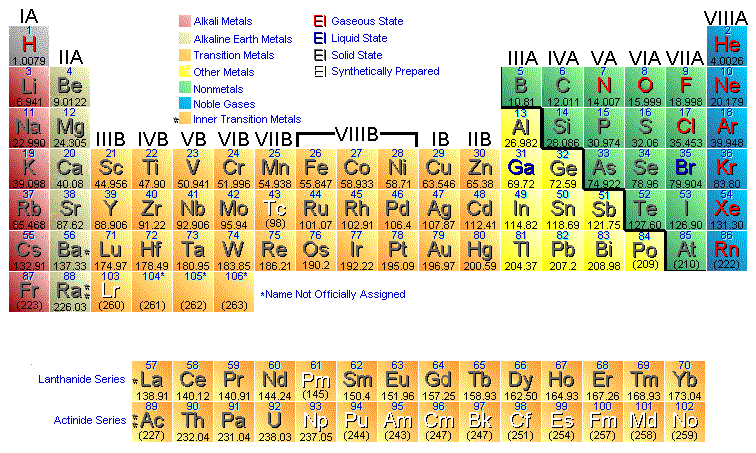
In the tranzition metals groups : group 3(IIIB) , group 4(IVB)...,group 7,8and 9(VIII B), group 11 (IB) , group 12(IIB)
Why groups 7,8,9 are all VIIIB?
And what does the "B" represent?
Why does the tranzition metals series start with III B and end with I B and II B?
And why group 13 is still IIIB?
And...in the groups 1,2,14,15,16,17,18 , what does the "A" represent ( I A, IVA , V A, VI A , VII A , VIII A)
In the tranzition metals groups : group 3(IIIB) , group 4(IVB)...,group 7,8and 9(VIII B), group 11 (IB) , group 12(IIB)
Why groups 7,8,9 are all VIIIB?
And what does the "B" represent?
Why does the tranzition metals series start with III B and end with I B and II B?
And why group 13 is still IIIB?
And...in the groups 1,2,14,15,16,17,18 , what does the "A" represent ( I A, IVA , V A, VI A , VII A , VIII A)
1 Answer
Well, first of all, groups 8, 9, and 10 are VIIIB, not 7, 8, and 9...
 http://knowthatplace.com/
http://knowthatplace.com/
If you just count the columns, then
The transition metals start at

