Does anything have a positive enthalpy of formation?
1 Answer
Feb 11, 2018
Plenty. But all elements in their elemental standard state have zero enthalpies of formation. You can only have positive values for non-elemental states.
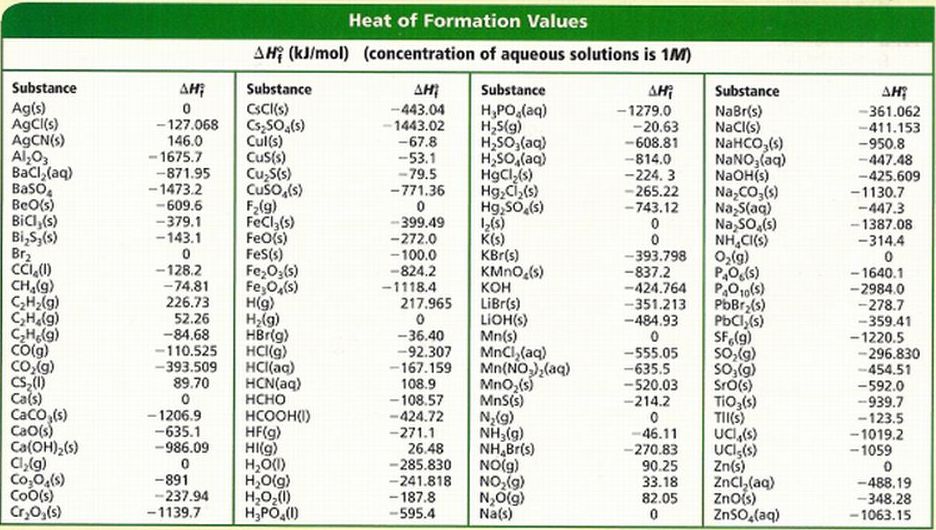 http://stahonorschemistry.weebly.com/
http://stahonorschemistry.weebly.com/
I spot
and these reactions are...
#"Ag"(s) + "C"(gr) + 1/2"N"_2(g) -> "AgCN"(s)#
#2"C"(gr) + "H"_2(g) -> "C"_2"H"_2(g)#
#2"C"(gr) + 2"H"_2(g) -> "C"_2"H"_4(g)#
#"C"(gr) + 1/4"S"_8("orthorhombic") -> "CS"_2(l)#
#1/2"H"_2(g) -> "H"(g)#
#1/2"H"_2(g) + "C"(gr) + 1/2"N"_2(g) -> "HCN"(aq)#
You should be able to explain why the formation of

