I already have, over here:
What is the shape of f-orbital???
Here are the radial density distributions of the #4s#, #4p#, #4d#, and #4f# orbitals:

From the above graph, the #4f# orbitals are the least effective at electron shielding, as they are the least penetrating orbitals in their quantum level; the radial electron density tapers off before getting near the nucleus, and so the #4f# electrons are usually not near the nucleus.
The lanthanide contraction occurs in part because the #6s# and #5s# electrons penetrate the core significantly and relativistically contract due to traveling close to the speed of light.
The #4f# and #5f# electrons shield poorly (the #5f# does a bit of a better job but not that much better), as seen below:

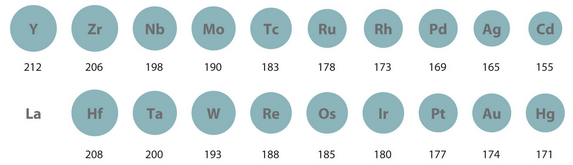
This can be observed in the 3rd row transition metals, which have only SLIGHTLY larger atomic radii than the respective 2nd row transition metals: