Write out the symmetry elements and operators for cyclopentane, and determine its point group?
1 Answer
I assume you mean a pentagon... if not, ask for the conformations...
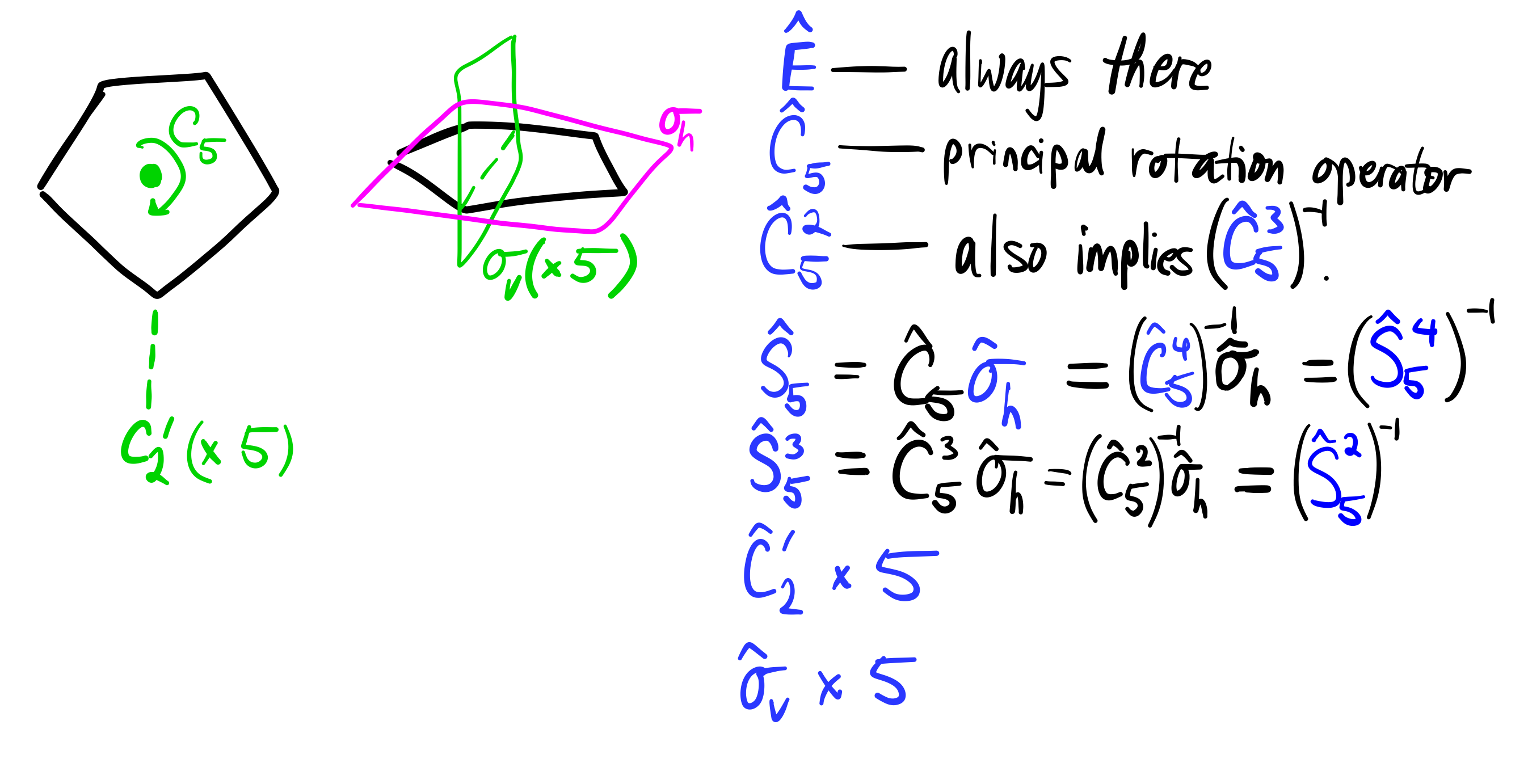
- Every molecule has an identity element
#E# , with corresponding operator#color(blue)(hatE)# . - This pentagon has a five-fold rotational axis element, i.e.
#C_5# axis on the#z# axis, and the corresponding#color(blue)(hatC_5)# operator .
If there's a
#hatC_5# , there's a#color(blue)(hatC_5^2)# (as you can do the same thing twice). Also,#color(blue)(hatC_5^3) = (hatC_5^2)^(-1)# is also included, and#color(blue)(hatC_5^4) = hatC_5^(-1)# is included. Why are they inverses?
- There is a horizontal mirror plane element
#sigma_h# with corresponding operator#color(blue)(hatsigma_h)# .
If there is both
#hatC_n# and#hatsigma_h# , then there also exists#hatS_n# , the improper rotation operator. Its corresponding individual elements have already been mentioned... and the operator is then#color(blue)(hatS_5)# .If there is
#hatS_5# , then there is#color(blue)(hatS_5^3)# . That means we also include#color(blue)(hatS_5^2)# as the inverse of#hatS_5^3# , and#color(blue)(hatS_5^4)# as the inverse of#hatS_5# .
-
There are also FIVE
#C_2'# rotation axis elements coplanar with the plane of the molecule, one of which is along the#x# axis. It corresponds to the#color(blue)(hatC_2')# operator, one for each element. -
There are lastly FIVE
#color(blue)(sigma_v)# vertical mirror plane elements perpendicular to the plane of the molecule (corresponding to the#hatsigma_v# operator), one of which is on the#xz# plane.
That gives
Upon knowing that there exist
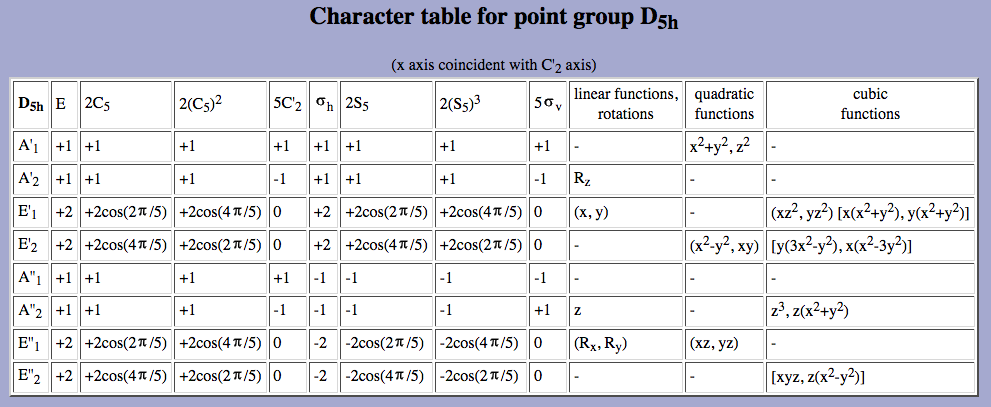
This is summarized in the character table: