Why do molecules with multiple bonds not considered to have more electron groups than molecules with single bonds?
1 Answer
Jan 15, 2018
Because molecular shape has only to do with the number of bonding directions.
All that is accomplished by having a multiple-bond is more electron-repulsion of surrounding bonds. Consider comparing these three molecules:
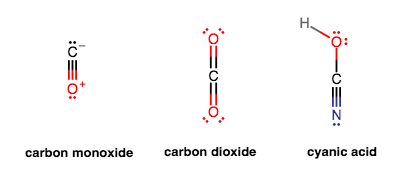
The bond angles around the carbon are
Either way, the number of bonding groups around the carbon do NOT change for all three cases. The molecular shape is still basically linear around carbon.

