Answer these questions about glycine?
#a)# Describe the structure and charge(s) of glycine below, at, and above #"pH"# #7.4# .
#b)# What is the #"pH"# of each form of glycine in separate aqueous solutions at body temperature, if the concentration is #"0.1 M"# for them initially? #K_(a1) = 4.57 xx 10^(-3)# , #K_(a2) = 2.51 xx 10^(-10)# .
1 Answer
Glycine is the simplest amino acid, containing two
#"H"# atoms on its#alpha# carbon:
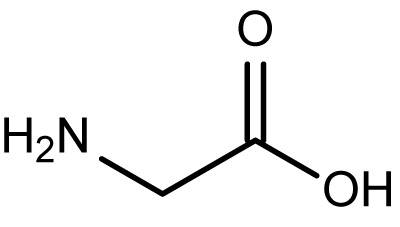
The carboxyl proton
#"pKa"# is near#2.2# , and the amine proton#"pKa"# is around#9.8# . Thus,
- Below
#"pH" ~~ 2.2# , the amine proton is ON, and the carboxyl proton is ON. This is the#"AA"^+# form.- At
#"pH" = 7.4# (body#"pH"# ), the amine proton is ON, and the carboxyl proton is OFF. This is the#"AA"^0# form.- Above
#"pH" ~~ 9.8# , the amine proton is OFF, and the carboxyl proton is OFF. This is the#"AA"^-# form.You can draw those by interpreting the notes above.
The
#"pH"# of#"0.1 M"# solutions of each of these forms is based on the#K_a# values. The most acidic form is#"AA"^+# and the most basic form is#"AA"^-# .With
#K_(a1) = 4.57 xx 10^(-3)# and#K_(a2) = 2.51 xx 10^(-10)# , the first one can be seen to belong to the carboxyl proton (why? It's higher, so it is ascribed to the more acidic proton). Treating glycine as a diprotic acid,
#"H"_2"Gly"^(+)(aq) rightleftharpoons "HGly"(aq) + "H"^(+)(aq)#
#"I"" "" ""0.1 M"" "" "" "" ""0 M"" "" "" "" ""0 M"#
#"C"" "" "-x" "" "" "" "+x" "" "" "" "+x#
#"E"" "(0.1-x)"M"" "" "" "x" M"" "" "" "x" M"# And so, write the mass action expression:
#K_(a1) = 4.57 xx 10^(-3) = x^2/(0.1 - x)# Naively using the small
#x# approximation,
#4.57 xx 10^(-3) ~~ x^2/0.1#
#=> x_0 ~~ sqrt(4.57 xx 10^(-3) cdot 0.1) = "0.0214 M"# That is not yet correct. We then generate our next guesses to converge onto the final
#x# :
#=> x_1 ~~ sqrt(4.57 xx 10^(-3) cdot (0.1 - 0.0214)) = "0.0190 M"#
#=> x_2 ~~ sqrt(4.57 xx 10^(-3) cdot (0.1 - 0.0190)) = "0.0192 M"#
#=> x_3 ~~ sqrt(4.57 xx 10^(-3) cdot (0.1 - 0.0192)) = "0.0192 M"# And so,
#["H"^(+)] = "0.0192 M"# , giving
#color(blue)("pH") = -log(0.0192) = color(blue)(1.72)# .The first conjugate base is a much weaker acid. Again, we treat this as in a separate solution:
#"HGly"(aq) rightleftharpoons "Gly"^(-)(aq) + "H"^(+)(aq)#
#"I"" "" ""0.1 M"" "" "" ""0 M"" "" "" ""0 M"#
#"C"" "" "-x" "" "" "+x" "" "" "+x#
#"E"" "(0.1-x)"M"" "" "x" M"" "" "x" M"# And so, write the mass action expression:
#K_(a2) = 2.51 xx 10^(-10) = x^2/(0.1 - x) ~~ x^2/0.1# Here, the small
#x# approximation is justified;#K_(a2)# #"<<"# #10^(-5)# . Thus:
#x = ["H"^(+)] = sqrt(2.51 xx 10^(-10) cdot 0.1) = 5.01 xx 10^(-6) "M"# And so,
#color(blue)("pH") = -log["H"^(+)] = -log(5.01 xx 10^(-6)) = color(blue)(5.30)# .Its conjugate base is obviously a base, and so, treating this as in ANOTHER separate solution, we need the
#K_b# .At body temperature,
#37^@ "C"# ,#K_w = 2.42 xx 10^(-14)# , so:
#K_b = K_w/K_a = (2.42 xx 10^(-14))/(2.51 xx 10^(-10)) = 9.64 xx 10^(-5)# This is still small enough for the small
#x# approximation.
#"Gly"^(-)(aq) + "H"_2"O"(l) rightleftharpoons "HGly"(aq) + "OH"^(-)(aq)#
#"I"" "" ""0.1 M"" "" "" "-" "" "" ""0 M"" "" "" ""0 M"#
#"C"" "" "-x" "" "" "" "-" "" "+x" "" "" "+x#
#"E"" "(0.1-x)"M"" "" "-" "" "" "x" M"" "" "x" M"# Just as before...
#K_b = 9.64 xx 10^(-5) = x^2/(0.1 - x) ~~ x^2/0.1# Thus, since
#["OH"^(-)] = sqrt(K_b["Gly"^(-)])# , the#["H"^(+)]# is:
#["H"^(+)] = K_w/(["OH"^(-)]) = (2.42 xx 10^(-14))/(sqrt(9.64 xx 10^(-5) cdot 0.1))#
#= 7.79 xx 10^(-12) "M"# This gives a
#"pH"# of
#color(blue)("pH") = -log(7.79 xx 10^(-12)) = color(blue)(11.1)#

