Identify the types of bonds (dative vs. covalent) indicated in the compound shown below?
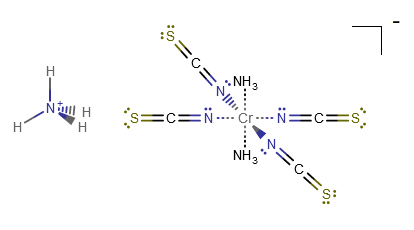
- Front Cr-N with
#""^(-)"NCS"#
- Rear Cr-N with
#""^(-) "NCS"#
- Top Cr-N with
#"NH"_3#
- The rear N-H in the top
#"NH"_3#

- Front Cr-N with
#""^(-)"NCS"# - Rear Cr-N with
#""^(-) "NCS"# - Top Cr-N with
#"NH"_3# - The rear N-H in the top
#"NH"_3#
1 Answer
The shown compound is
That
#"Cr"-"N"# bond is a dative bond, which is primarily based on the donation of electrons from the nitrogen into the chromium. Dative bonds are based on both electrons coming from the same atom.The wedge indicates that it faces towards us.
Same as earlier.
The only difference is that it is facing away from us, trans from the previous one.
Almost the same as the first one.
The only difference is that besides the fact it is in the plane of the screen and along the
#z# axis,#"NH"_3# is the stronger-field ligand, so there is more direct overlap of nitrogen's atomic orbital with chromium's#d_(z^2)# atomic orbital than#""^(-)"NCS"# has with chromium's#d_(x^2-y^2)# .[It still doesn't change the type of bond.]
That is a regular
#"N"-"H"# covalent bond in#"NH"_3# , a polar molecule.The dash again just means that the bond is facing away from us, but it does not differ from the solid
#"N"-"H"# bond on the same ammonia ligand.

