Why does the reaction with #("CH"_3)_3"Br"# proceed with an #"S"_N1# and/or #"E"1# mechanism? What does it have to do with steric hindrance?
1 Answer
It can be considered as basically spatial crowding.
Steric hindrance is a kinetic factor that limits the ease to which a nucleophile (electron pair donor) can approach an electrophile (electron pair acceptor).
As an example, consider the reaction seen below, which one might hope is
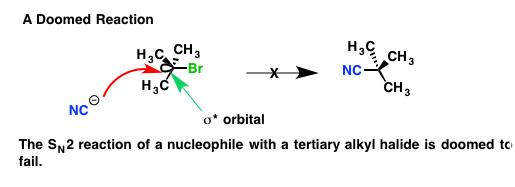
Here, the nucleophile is cyanide (
We should predict that reaction does not work via an

(LEFT: steric hindrance; RIGHT: reduced steric hindrance)
That reduces the ease to which a successful collision can occur between two reactants, and slows down the first step in a given substitution mechanism (making it the rate-limiting step).
Hence, this reaction proceeds more easily as a first-order mechanism (e.g.
The rate law for this could then be approximated as first-order based on the slow step, since it dominates the extent of the reaction time:
#r(t) ~~ k_1["Br"^(-)]#
(Depending on the choice of solvent, either

