Question #8533e
1 Answer
I got
You should look up this sequence of reactions to understand these more:
- Friedel-Crafts acylation
- Aromatic bromination (reactions of substituted benzenes)
- Wolff-Kishner reduction (in base)
Beyond that, I can go into more detail below.
The overall synthesis would be:
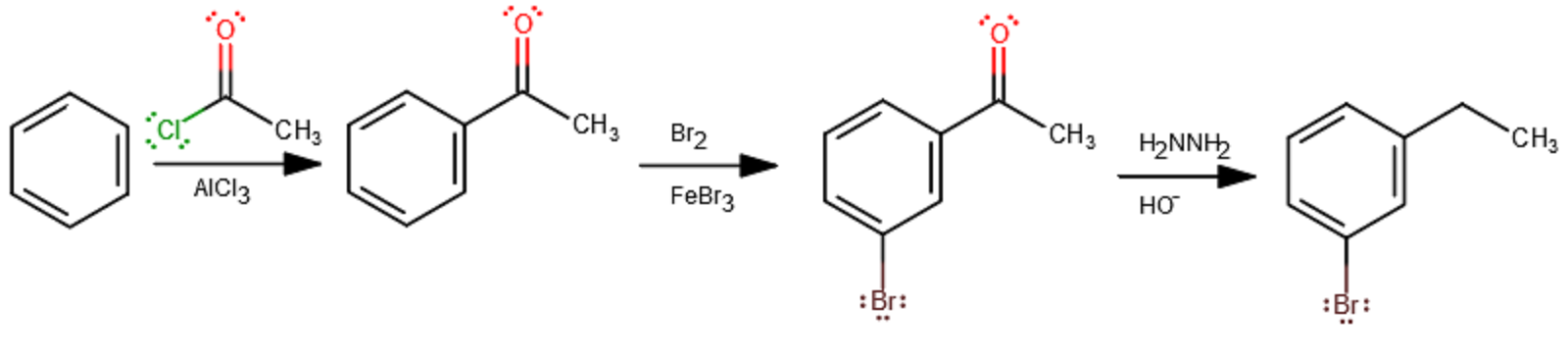
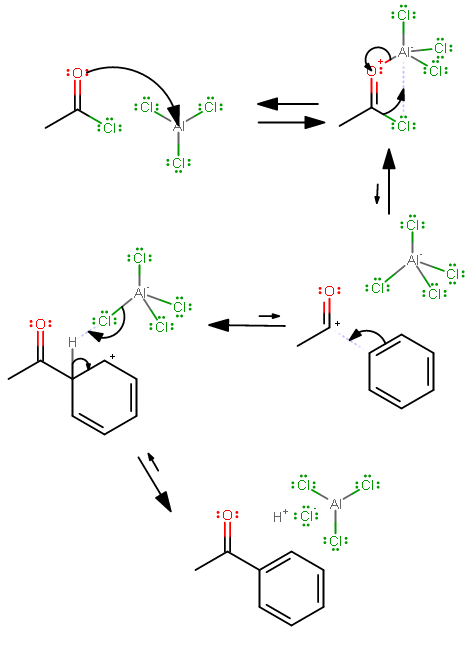
The Friedel-Crafts acylation turns the acetyl chloride into an electrophile using a strong Lewis acid,
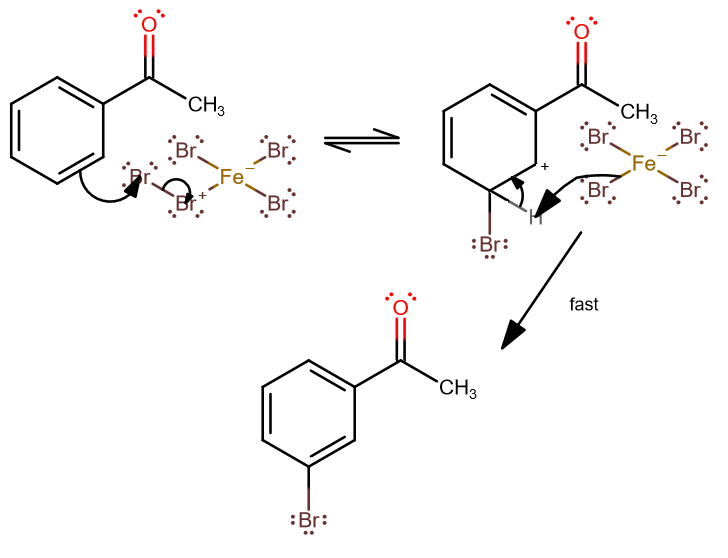
The aromatic bromination is influenced by the acyl group, which is a meta director. The
The Wolff-Kishner mechanism is quite long... It essentially turns a ketone into an alkane (removing the carbonyl) and releases
Thus, we obtain the product given for

