What is the hybridization of copper in tetraamminecopper(II) in water? Why is it NOT #sp^3#, and how do you prove this using Group Theory?
1 Answer
It would be
By the way, a great deal of this answer uses the concept of symmetry elements in chemical group theory. You should read that first if you don't want to be too confused.
GEOMETRY CONSIDERATIONS
Well, tetramminecopper(II) is square planar (and not tetrahedral). How do I know? Well, in water, as it must be (why?), it is more accurately represented as:
#["Cu"("NH"_3)_4("H"_2"O")_2]^(2+)#
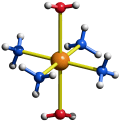
which is an octahedral complex. Without the waters in consideration, we thus have a square planar complex. As such, since this is not tetrahedral, this is not
SYMMETRY CONSIDERATIONS
This complex belongs to the
- It has an identity element
#E# , which is just there for completeness. - It has a
#C_4(z)# principal rotation axis perpendicular to its plane (i.e. rotate#90^@# and an indistinguishable configuration results). - It has (at least) one
#C_2'# rotation axis coplanar with the compound's plane, but perpendicular to the#C_4# axis. That is, it is on the#xy# plane, along the axes. - It has (at least) one
#C_2''# rotation axis on the#xy# plane, just as the#C_2'# axes are, but bisecting them instead. - It has a
#sigma_h# horizontal mirror plane, coplanar with the compound's plane. - It also has vertical mirror planes
#sigma_v# that line up with the coordinate axes (perpendicular to#sigma_h# ), and dihedral mirror planes#sigma_d# that bisect them. - It has the inversion element,
#i# .
This means it follows this character table:
OBTAINING THE REQUIRED ORBITAL SYMMETRIES
To determine what the hybridization COULD be systematically, we generate a so-called reducible representation
To do this, operate on the complex using each operation, and examine how the
- Unmoved outer atoms contribute
#1# . - Outer atoms that moved from their original position contribute
#0# .
#" "" "" "" "E" "C_4(z)" "C_2(z)" "C_2'" "C_2''" "i" "S_4" "sigma_h" "sigma_v" "sigma_d#
#Gamma_(NH_3) = " "4" "0" "" "" "0" "" "2" "" "0" "" "0" "" "0" "4" "" "2" "0#
Skipping some steps, we reduce this using a reduction calculator for
#ul(Gamma_(NH_3)^("red") = A_(1g) + B_(1g) + E_u)#
The point of this was to see which symmetries the
EXTRACTING COMBINATIONS OF HYBRID ORBITALS
Examine the last two columns of the character table, match up
#A_(1g): x^2+y^2, z^2# includes the#s# and#d_(z^2)# orbitals, respectively.#B_(1g): x^2 - y^2# includes the#d_(x^2-y^2)# orbital.#E_u: (x,y)# includes the#p_x# and#p_y# orbitals at the same time.
These are the irreducible representations we got earlier. It turns out that this set of irreducible representations allows the following possibilities for orbital hybridizations:
#psi_"hybrid" = c_1overbrace(d_(z^2))^(A_(1g)) + c_2overbrace(d_(x^2 - y^2))^(B_(1g)) + c_3overbrace((p_x + p_y))^(E_u)# #" "bb((i))#
#psi_"hybrid" = c_1overbrace(s)^(A_(1g)) + c_2overbrace(d_(x^2 - y^2))^(B_(1g)) + c_3overbrace((p_x + p_y))^(E_u)# #" "bb((ii))# where the
#c_i# are arbitrary constants such that#int_"allspace" psi^"*"psi d tau = 1# .
This means we could have had either
RATIONALIZING WHICH HYBRIDIZATION IS MORE REASONABLE
Since the
Thus, we expect

