As it is aligned along coordinate axes, the #d_(x^2-y^2)# usually forms #sigma# bonds in preference to #pi# bonds, seeing as how ligands bond along the coordinate axes as the internuclear axes.
An example is in square planar transition metal complexes...
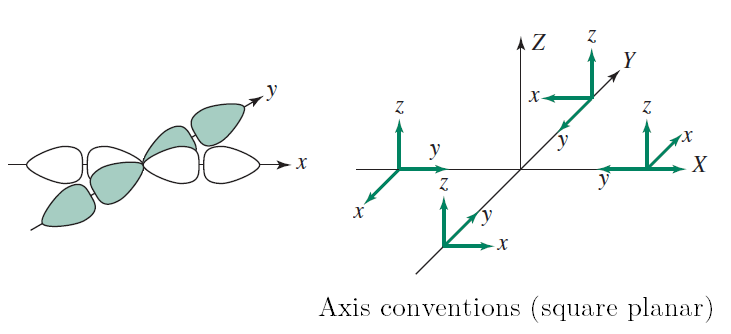
...or in octahedral transition metal complexes:
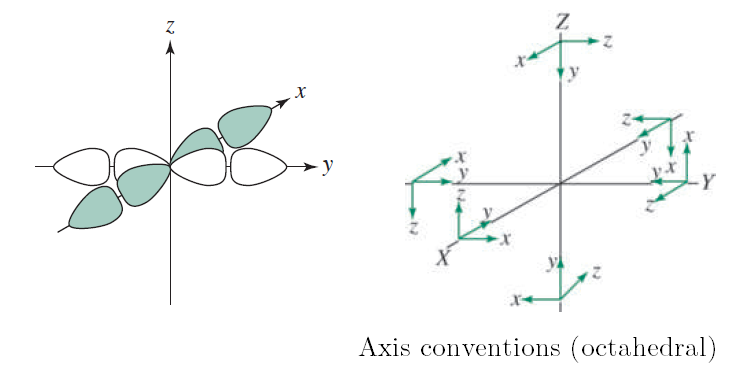
One "exception" (among others) is trigonal bipyramidal transition metal complexes, where the #d_(x^2-y^2)# does not quite provide a direct #sigma# interaction. In that case, it only is a partially direct overlap and not an ideal #sigma# bond.
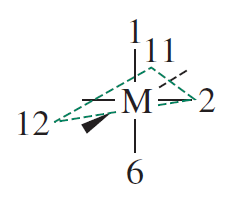
The #d_(x^2-y^2)# orbitals would lie along the coordinate axes defined by ligand "#2#" and the wedge bond, whereas the ligands would lie on the corners of the dashed triangle.
See this answer for further detail on the #d_(x^2-y^2)# orbital.
On the other hand, the #d_(xy)#, #d_(xz)#, and #d_(yz)# tend to form #pi# bonds, and rarely #delta# bonds.
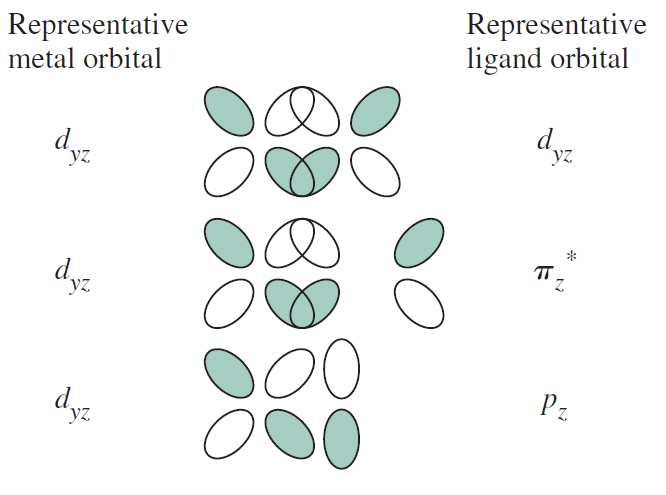
One note here is that usually the internuclear axis is the #z# axis, but with some exceptions.
The above diagram for octahedral complexes uses the convention that in polyatomic compounds, the #bby# axis is the internuclear axis in the coordinates of the outer (non-central) atoms.

