How do you find the effective nuclear charge of lanthanides?
1 Answer
You do not have to understand this throughout your chemistry curriculum (or at least, I never had to), because it is the result of multiple complicated competing trends. The effective nuclear charge in general is not predictable for the Lanthanides.
Due to scalar relativistic effects, the
Slater's rules incorrectly predict something like the following for
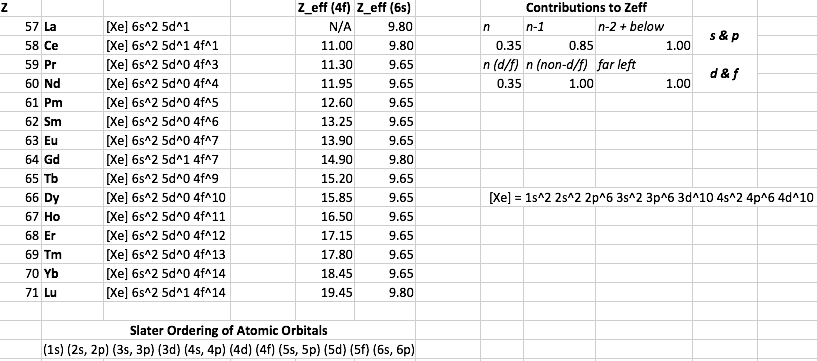
However, the atomic radii are all over the place:

This is likely due to spin-orbit relativistic effects, where the
Normally, we use

