What is the molecular electron configuration of #"O"_2#?
1 Answer
#(sigma_(1s))^2(sigma_(1s)^"*")^2(sigma_(2s))^2(sigma_(2s)^"*")^2(sigma_(2p_z))^2 (pi_(2p_x))^2(pi_(2p_y))^2(pi_(2p_x)^"*")^1(pi_(2p_y)^"*")^1#
You should read the explanation on how we came up with this, below.
When determining the molecular orbital (MO) configuration of a homonuclear diatomic molecule like
We had for each
#1s^2 2s^2 2p^4#
The AOs of
LINEAR COMBINATION OF ATOMIC ORBITALS
The number of AOs in equals the number of MOs out.
Any
- In-phase to form
#sigma_(ns)# /sigma bonding MOs - Out-of-phase to form
#sigma_(ns)^"*"# antibonding MOs
The
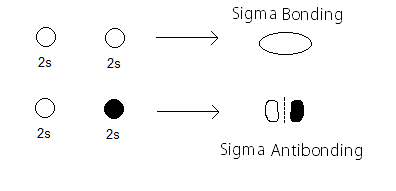
with a partial MO diagram like this:
Along the internuclear (
- In-phase to form
#sigma_(np_z)# bonding MOs - Out-of-phase to form
#sigma_(np_z)^"*"# antibonding MOs
Here's how the
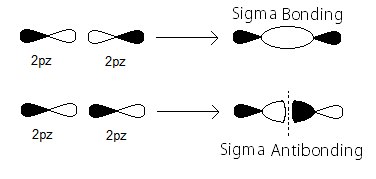
The partial MO diagram depiction of those is similar to the
Finally, the
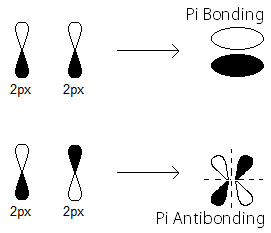
For homonuclear diatomics after
This portion of the MO diagram (the
Overall, the MO diagram is therefore like this:
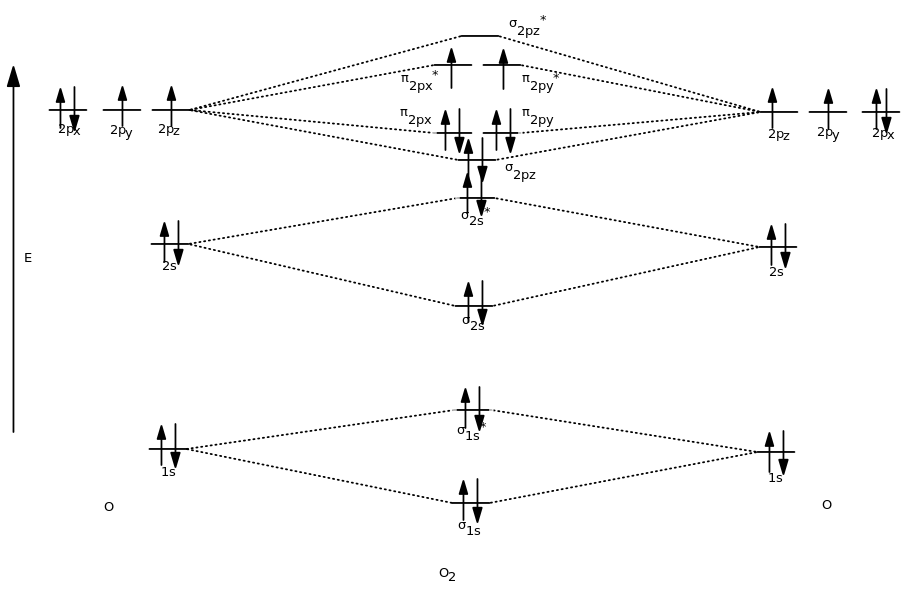
The MOs were filled just like the AOs would be filled according to the Aufbau Principle, Hund's Rule, and the Pauli Exclusion Principle.
So, write the MO configuration based on this diagram. Start with the lowest-energy orbital, and indicate the electrons in each kind of orbital, just like atomic electron configurations.
You should get:
#color(blue)((sigma_(1s))^2(sigma_(1s)^"*")^2(sigma_(2s))^2(sigma_(2s)^"*")^2(sigma_(2p_z))^2 (pi_(2p_x))^2(pi_(2p_y))^2(pi_(2p_x)^"*")^1(pi_(2p_y)^"*")^1)#

