When the temperature doubles from thermodynamic standard temperature, which of the following most completely describes how the gas sample increases in volume?
#a)# the number of molecules increases.
#b)# the force with which the particles collide with the container walls increases.
#c)# the collisions with the walls occur more often.
#d)# the average distance between molecules increases because the kinetic energy of the sample increases.
1 Answer
It's the last one.
Well, let's think about it conceptually...
1) No, because it's not relevant. You would still have the same number of atoms regardless.
2) No, because this describes the change in pressure.
Striking a wall with some force implies that you have a force per unit area, which is the definition of pressure,
#P = F/A# .3) No, for the same reason as (2).
Also, it doesn't matter that it does so more often. That should be obvious since the gas particles move faster.
4) Not entirely correct, but for this question it probably is the correct choice.
The average kinetic energy increases, not the kinetic energy. The kinetic energy of one molecule might be lower than another's. It might also be higher than another's. We don't really know which, so we say the following.
You have a kinetic energy distribution and gas speed distribution in the form of a Gaussian curve, described as the Boltzmann distribution.
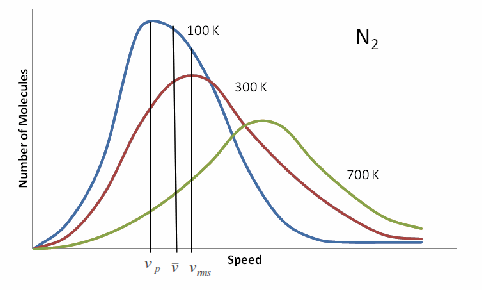
What this means is that you have a population of gas molecules that each have a certain kinetic energy, and they form a distribution that looks something like the above.
That aside, this answer choice discusses the average distance between molecules increasing, which is talking about an increase in volume.

