Which electrons are easiest to give away from an atom? Of these, are lone pairs or bonding pairs of electrons easier to give away? What orbitals are they in?
1 Answer
Don't think too hard about this.
(1)
The outermost electrons in a molecule are easiest to access and consequently give away, because... well, they're on the outside.
These are, by definition, the valence electrons of the atom of interest within the molecule.
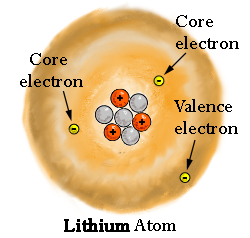
(2)
The lone pairs can be donated most easily because they are not confined into a bond. For example,
(3)
Next, they must also be the highest in energy in the molecule, because then they are most willing to leave so that the molecule is stabilized.
That means they are in the highest-energy occupied molecular orbital, or HOMO (whereas LUMO means lowest-unoccupied molecular orbital).
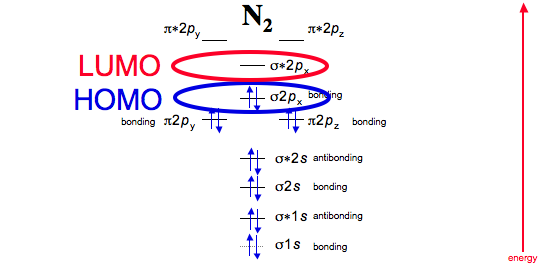
And finally, the electrons in action:
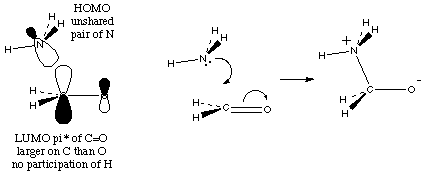
So, putting that together:
- Valence electrons on the atom of interest.
- Usually, they are lone pairs that are not being used to bond at the moment.
- These should also be inside the highest-energy occupied molecular orbital.

