How do you draw the MO diagram for #"O"_2# from scratch?
1 Answer
To draw a molecular orbital (MO) diagram, you need to consider which atomic orbitals (AOs) the molecule has.
ATOMIC ORBITAL DIAGRAM FOR OXYGEN ATOM
Oxygen atom is on period 2, so it has access to its
(The
Then, note that oxygen atom has
- Hund's rule (maximize the number of unpaired electrons).
- The Aufbau principle (fill orbitals from lowest to highest energy).
- The Pauli Exclusion Principle (paired electrons are opposite in "spin", or direction).
If you are unsure about any of these rules or principles, you should ask for further clarification, as I assume you already are familiar with these.
At this point, we have the AO diagram as follows for both oxygen atoms:

DETERMINING ATOMIC ORBITAL INTERACTIONS
Next, consider which orbitals can interact with each other. That means which ones can overlap to create an effective bond.
For a homonuclear diatomic molecule like
These interactions generate what are called molecular orbitals, and they will conserve the number of orbitals. That means with
TYPES OF MOS TO DRAW
Next, recall that there exist bonding (
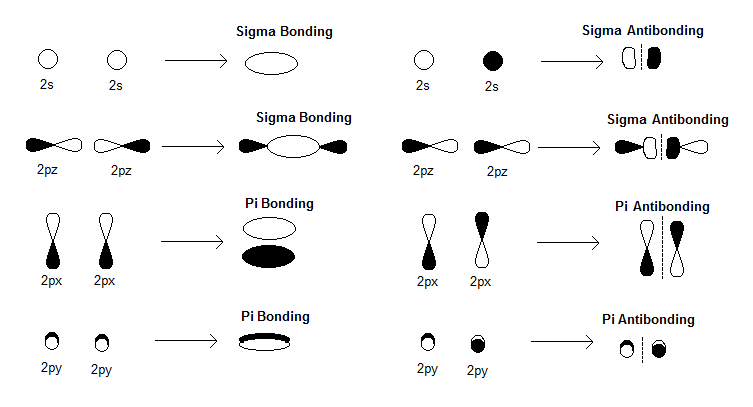
When we consider these interactions, we'll see the
and the slightly more complicated
With
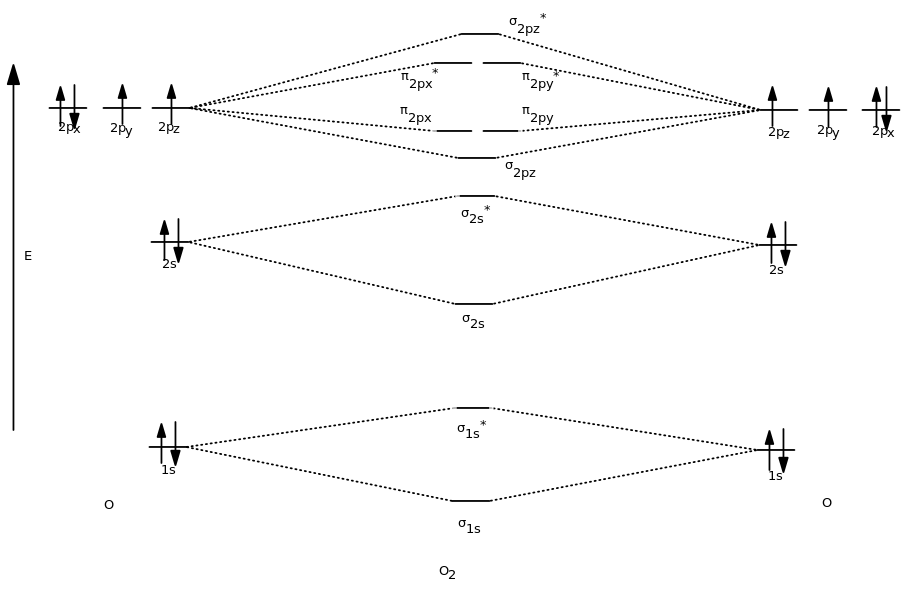
So, when we combine what we see here into the full diagram, we first get:
And then when we fill the MOs with the
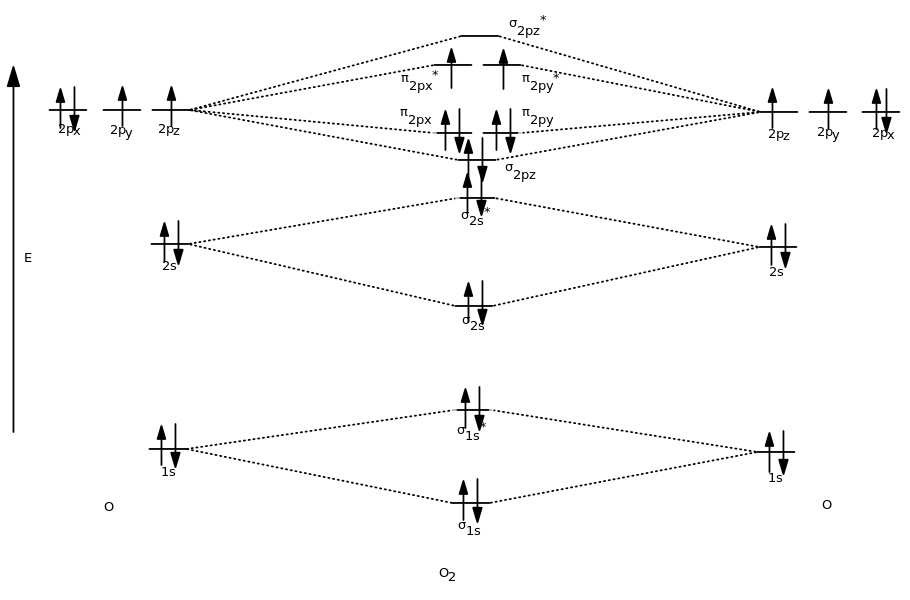
Remember that that was for
- To modify the diagram for
#"O"_2^(+)# , simply remove one of the highest-energy electrons. - To modify the diagram for
#"O"_2^(-)# , simply add one electron to the highest-energy MO.
If you have any questions still, and if this is still confusing, just ask.

