Due to what is ice less dense than water?
1 Answer
Feb 16, 2018
Due to hydrogen-bonding!
Hydrogen-bonding leads to water forming a bulk interaction that leaves it more dense than the crystal structure of ice.

As we know,
#rho_"ice" = "0.9167 g/cm"^3# at#0^@ "C"#
#rho_"water" = "0.9998425 g/cm"^3# at#0^@ "C"# so water is denser by a factor of about
#1.091# .
That leads to a solid-liquid coexistence curve with a left-tilt, i.e. increasing the pressure a lot forms water, not ice.
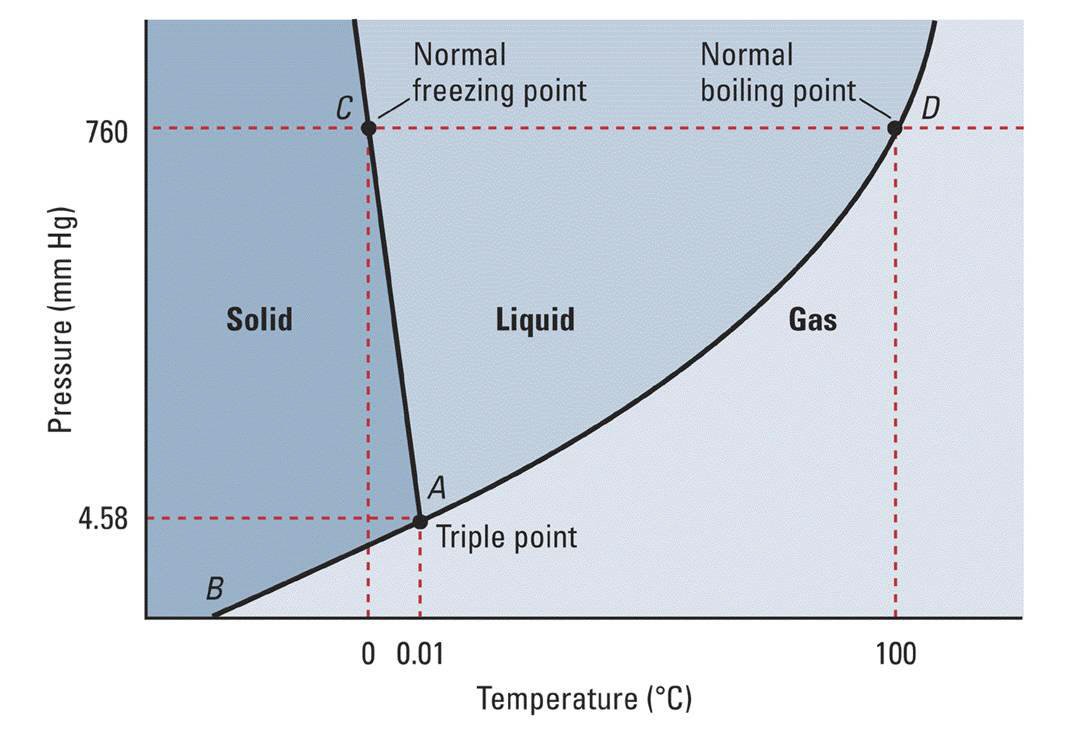
The slope of that curve is approximately given by the Clapeyron equation for the solid-liquid phase equilibrium:
#(dP)/(dT) = (DeltabarH_(fus))/(T_fDeltabarV)#
- Since
#barV prop 1/rho# , and the density decreases going from water to ice,#DeltabarV# is positive #T_f# (being in#"K"# ) is positive.- Freezing removes heat, so
#DeltabarH_(fus)# is negative.
#(dP)/(dT) = ((-))/((+)(+)) = (-)#
Thus,

