What is the best way to determine if an organic molecule is really aromatic?
1 Answer
More often than not, knowing how to check via resonance structures for the full
The conditions for aromaticity can be summarized like so:
A planar ring with
#\mathbf(4n + 2)# #pi# electrons within the#pi# system, where every atom in the ring can participate in the delocalization of the#pi# electrons, is aromatic.
On the other hand, something antiaromatic follows the
Therefore, you have to prove aromaticity before you can assert antiaromaticity.
There is no clear-cut, "best", "most efficient" test for aromaticity, but in general it usually works to check the resonance structure for whether or not the
CHECKING
Proper resonance structures are usually, but not always, a good indication of aromaticity.
You have to understand, from examining the electron geometry, which electrons are truly delocalized within the ring, and which ones are not. That matters.
For example, consider pyridine, which I'm going to tell you ahead of time is aromatic.
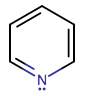
MISTAKEN USAGE OF HÜCKEL'S RULE DOES NOT DISPROVE AROMATICITY
Count how many
If you counted
Why? Because they are perpendicular to the rest of the ring, and thus are localized outside of the ring. They do not participate in the resonance structure and are NOT going to count in the
PYRIDINE'S
If you proceed to check the resonance structures, you can see that the
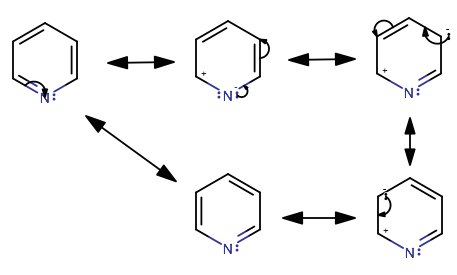
That satisfies probably the most difficult condition for aromaticity.
Finally, it is obvious that pyridine is cyclic (it's a ring). Pyridine is also planar due to its depiction having alternating double bonds (
SOMETIMES RESONANCE STRUCTURES FAIL
Resonance structures don't always work, so you should always consider all of the conditions for aromaticity.
For instance, sometimes you may think "hey, this is fully conjugated around the ring. It's aromatic, right?" However, there are exceptions.
One such exception is 1,3-cyclobutadiene.
I won't go too much into detail on this, but this molecule is neither aromatic nor antiaromatic, not only because of its
I've written a more in-depth discussion of this here:
http://socratic.org/questions/why-is-1-3-cyclobutadiene-not-aromatic
CHALLENGE: Try drawing the resonance structures for the 1,3-cyclopentadiene anion (deprotonated on its only

