How to predict hydroboration-oxidation?
1 Answer
Ah, I thought you had asked for hydrobromination.
Okay, so I'm assuming you mean how one could predict PRODUCTS of hydroboration.
Simple alkene hydroboration is ultimately an anti-Markovnikov process.
A typical hydroboration taught in Organic Chemistry can use any equivalent way of expressing the following reagents:
1)
#"BH"_3# complexed with#"THF"#
2) ...followed by#"NaOH"(aq)# and#"H"_2"O"_2#
The mechanism is as follows for an alkene (Organic Chemistry, Bruice):
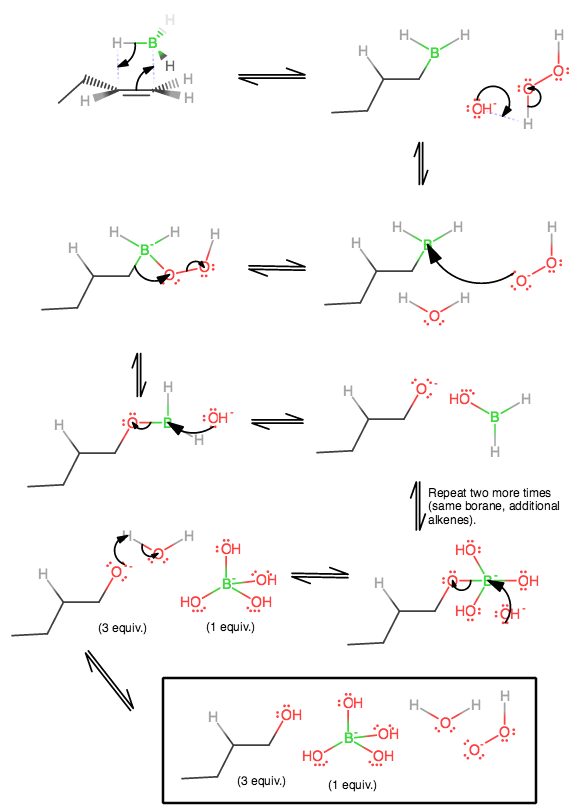
1) This starts with the interaction of the
The left carbon is the one accepting electrons into its antibonding
This is important. Because the left carbon has a methyl and the right carbon has two hydrogens, the hydrogen adds to the side with LESS hydrogens, rather than more, correlating with anti-Markovnikov addition. This ensures the addition of the hydroxide on the LESS substituted carbon at the end.
2) Now, hydroxide must equilibrate with hydrogen peroxide, because the pKa of hydrogen peroxide is about 11.2, but that of water is about 15.7, so water is a weaker acid. The equilbrium lies on the side of the weaker acid.
3) Peroxide, being a good nucleophile now, can backside-attack boron's empty
4) A kinda freaky thing happens where the electrons shift from the
5) At this point, boron's
6) Thus, we form our alkoxide precursor to the alcohol. Now we repeat steps 1-4 once, move on to step 7, then go back to do steps 1-4 one more time, and then do 7 again.
7*) Here, we do step 5 again, except using the hydroxyborane that formed after performing step 6 with updated versions of the same borane.
8) Finally, we can finish this mechanism when the alkoxides acquire protons from the leftover water (remember, the
Ultimately, you end up with hydroxide on the LESS substituted carbon, and hydrogen on the MORE substituted carbon, an anti-Markovnikov addition.

