Cyclohexene to ethyl cyclohexyl acetylene?
1 Answer
Oct 5, 2015
I'm assuming you mean a synthesis process. Well, I would start with either cyclohexene or bromocyclohexane. It just so happens that bromocyclohexane has cheaper useful purchase options from Sigma Aldrich (about $30 for 100 g, rather than about $50 for 500 mL), but theoretically/educationally, it's fine either way.
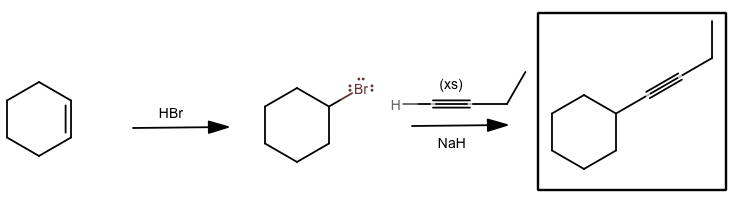
The HBr reaction is one of the first reactions you learned.
The NaH reaction plucks the proton off of the terminal alkyne and then that alkynyl anion acts as a nucleophile. If you add excess deprotonated alkyne, you should get the product you want. Specify excess with "xs".

