How do I correspond the structure of #"SO"_2# to a molecular orbital diagram?
1 Answer
Sulfur wants
- Putting
#4# for each of two double bonds uses up#8# . - You can then put
#4# electrons on each oxygen and the last#2# on the sulfur as a lone pair. Sulfur can be hypervalent (cf.#"SF"_6# ), but oxygen cannot because sulfur can access its#3d# orbitals (it's on the third period/row).
If we place this structure on the xy-plane, then the z-axis comes out towards us.
Sulfur has one
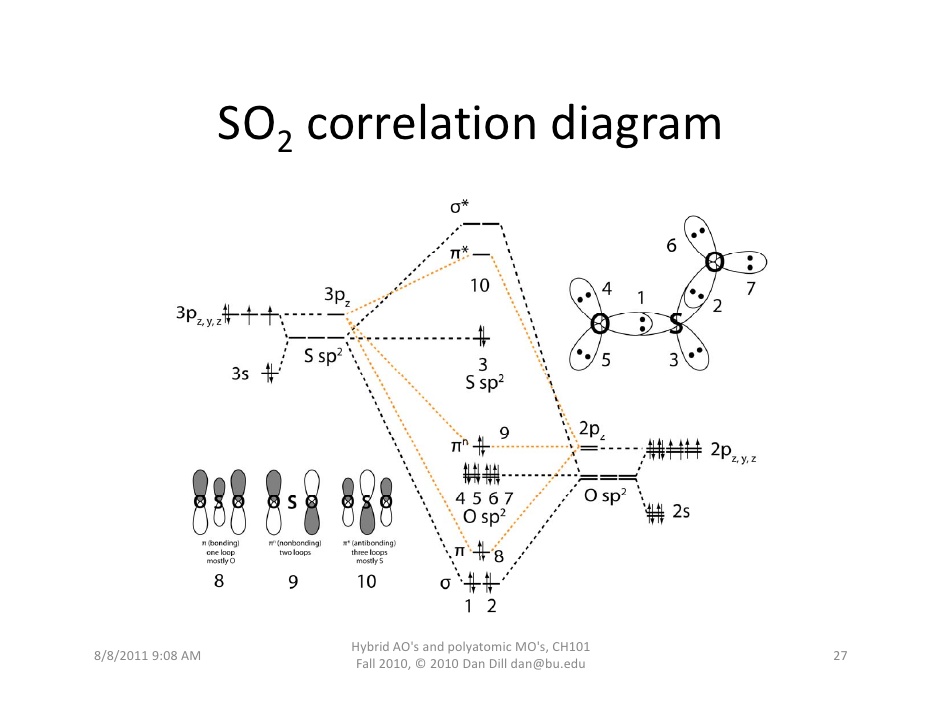
If you stare for a while at this diagram, which shows only the
SIGMA BONDING/ANTIBONDING ORBITALS
- The
#2s# and#2p# orbitals on oxygen mix to produce hybridized#sp^2# AOs for#sigma# bonding (the hybridized orbitals are indicated by the "#"O"# #sp^2# " label on the far right of the diagram) that are intermediate in energy between the original#2s# and#2p# AOs. - The
#3s# and#3p# orbitals on sulfur mix to produce hybridized#sp^2# AOs for#sigma# bonding (the hybridized orbitals are indicated by the "#"S"# #sp^2# " label on the far left of the diagram) that are intermediate in energy between the original#3s# and#3p# AOs. - Two of the
#sp^2# AOs (#2s + 2p_x + 2p_y# ) from oxygen can overlap head-on with two of the#sp^2# AOs (#2s + 2p_x + 2p_y# ) from sulfur to form one#sigma# bonding and one#sigma^"*"# antibonding MO. - The
#sigma# bonding MOs are labeled 1 for the left#"S"-"O"# #sigma# bond, and 2 for the right#"S"-"O"# #sigma# bond. The antibonding MOs are empty.
As a result, we should see that we have accounted for the
So far, our electron count is 2 x 2 = 4.
LONE PAIRS OF ELECTRONS (NONBONDING ORBITALS)
For the lone pairs of electrons, we examine the nonbonding MOs that have a dashed line connected to one atom's AOs but not the other's AOs. From the structure above, we should examine the diagonal orbitals, which have mixed
- One of sulfur's
#sp^2# hybrid AOs aren't overlapping with one of oxygen's#sp^2# AOs. That is the lone pair of electrons that sulfur has, and you can tell by noticing how the MO diagram shows the black dashed-line contribution from only the#sp^2# AOs of sulfur. - This is orbital 3 and is labeled as the nonbonding "
#"S"# #sp^2# " MO in the middle of the diagram, below MO 10. - There are
#sp^2# orbitals of oxygen that didn't overlap with the#sp^2# orbitals of sulfur. Those account for the two lone pairs of electrons on each oxygen, and you can tell by noticing how the MO diagram shows the black dashed-line contribution from only the#sp^2# AOs of oxygen. - These are MOs 4, 5, 6, and 7, labeled "
#"O"# #sp^2# ", and they are right below MO 9.
So far, our electron count is (2 x 2) + 2 + (2 x 4) = 14.
PI BONDING/ANTIBONDING/NONBONDING ORBITALS
Lastly, let us examine the
- The gist is that two
#\mathbf(pi)# bonds are made between the#2p_z# AOs of each oxygen and the#3p_z# of the sulfur. That makes sense since we had defined the axis coming towards us as the z-axis, the#2p_z# and#3p_z# orbitals both lie along that axis, and we hadn't considered those orbitals yet. They can overlap side-on to form#pi# bonds.
(I believe they are labeled as MOs 8 and 9 on the MO diagram, but I am unsure why 9 is nonbonding when in fact four
And our final, total electron count is (2 x 2) + 2 + (2 x 4) + (2 x 2) = 18, as predicted in the beginning.