Why do certain atoms emit certain colors of light? How do they get the energy to do so, and what kinds of particles are involved in this process?
2 Answers
Electrons, & photons too!
Explanation:
When atoms are heated, electrons will move from their ground state (lower energy level) to a higher energy level. This process is called excitation. It requires energy.
Once the electrons are at higher energy levels, they don't stay there forever. They will eventually fall back down to their original energy level (ground state).
As electrons move from higher energy levels to lower energy levels a photon (particle of light) will be given off. This is the process of emission. The photons will have different wavelengths and frequencies, this makes photons of different energies produce different colors of light.
Here are some videos of flame tests for different elements which demonstrate this concept.
Video from: Noel Pauller
Video from: Noel Pauller
Hope this was helpful.
Heated compounds have acquired energy in the form of thermal energy. This thermal energy, if it is enough, excites electrons in any major atom in a compound or any singular lone atom so that the compound reaches one of its accessible excited states. This event is called an electronic excitation, and it leaves the compound less stable than in its ground state.
That causes the compound to want to release energy to restore its stability, and it does so most easily by letting electrons that are in high-energy orbitals drop down to low-energy orbitals. This electronic relaxation emits energy in the form of light.
Depending on the energy change from one emission, the transition corresponds to specific wavelengths of light and thus specific colors. For example, here's sodium's energy level diagram:
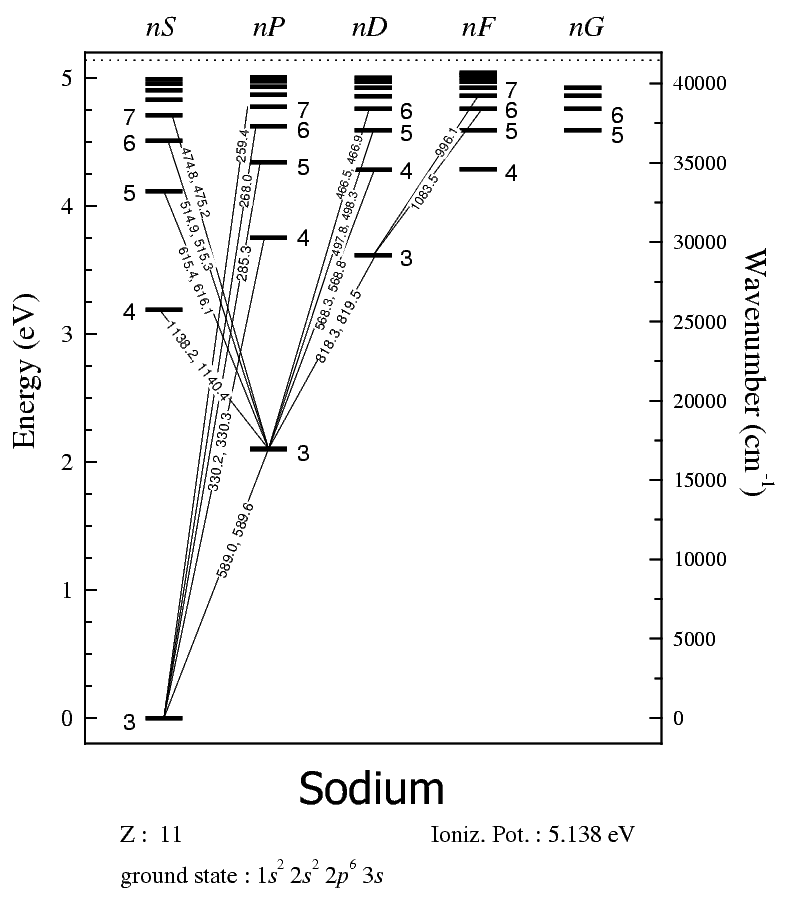
How you read this diagram is that the numbers next to the energy levels are the quantum number
So, the electronic excitation has a single electron going from the
The electronic relaxation in


