What do you think causes emission of light of various colors?? How do energy and wavelength relate as factors?
1 Answer
Emission of light of various colors from atoms is dependent on the energy gap between the occupied and unoccupied orbitals that participate in an electronic excitation. Higher energy, shorter wavelength.
Once an electron gets excited from the highest-energy occupied molecular orbital (HOMO) to the lowest-energy unoccupied or partially unoccupied molecular orbital (LUMO), it gets into an excited state (excitation... excited state... makes sense).
In the first excited state (only one promoted electron), the molecule is less stable, and wants to become more stable. The easiest way to do this is to let the electron fall from the LUMO to the HOMO because they are closest in energy. Then the atom achieves its ground-state.

For example, here is a table from Physical Chemistry: A Molecular Approach that displays sodium's spectroscopic data.
That corresponds to:
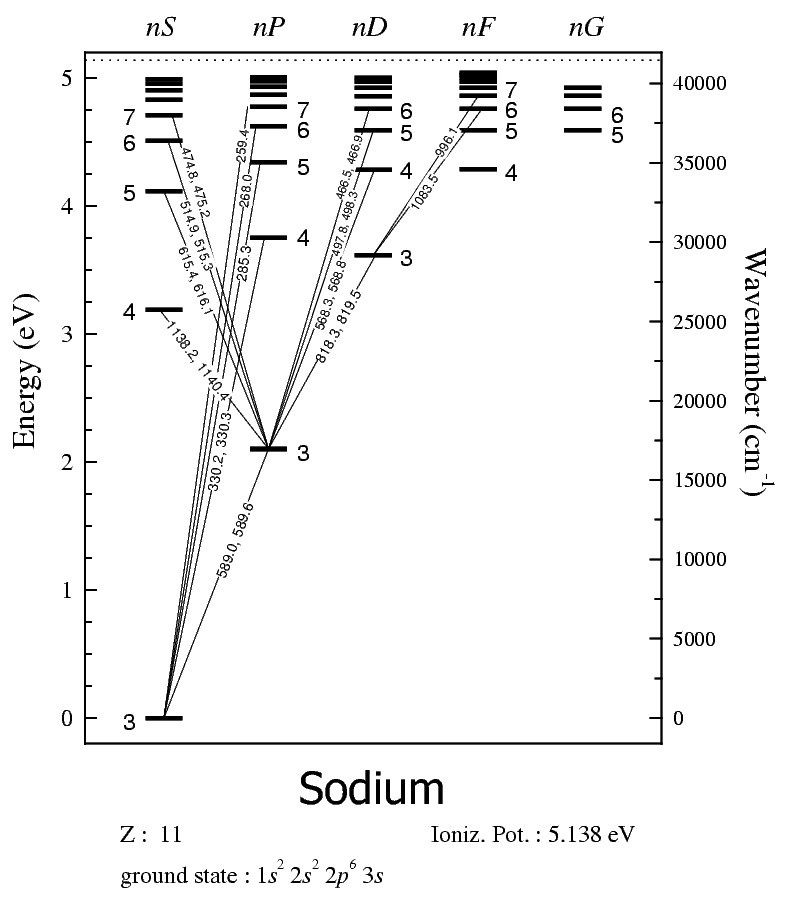
...the first and second 589 line here.
The yellow sodium emission line that we'll look at is from the doublet S-1/2 (
#lambda = c/nu = 1/(tildenu)# where
#c ~~ 2.998792458xx10^10 "cm/s"# (the speed of light),#nu# is frequency in#"s"^-1# ,#tildenu# is the energy in wavenumbers (#"cm"^-1# ), and#lambda# is wavelength. We want#lambda# in#"nm"# .
So all we do is reciprocate the energy in wavenumbers to get it in
Therefore,
#lambda = 1/(16956.183 cancel("cm"^-1))*(10^7 "nm")/cancel("cm") = color(blue)(589.75537 "nm")#
Since the speed of light is known to at least 8 sig figs, that is our answer, to that many decimal places. This wavelength indeed corresponds to yellow light.

Therefore, that electronic transition up prepares sodium to emit yellow light once it releases energy for the electronic relaxation from the
(The closeness of the two

