When do molecules peak out on making bonds?
1 Answer
My guess is that you're asking why we don't get quadruple bonds easily, and almost never get more than that (yes, a quintuple bond exists in some Chromium compounds!).
NORMAL CIRCUMSTANCES
In normal circumstances, molecules tend to "peak out", making triple bonds. That usually corresponds to the idea that the greater the number of
The more
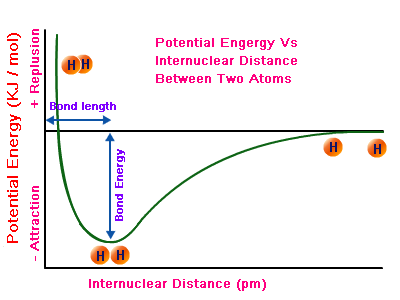
Essentially, a triple bond is shorter than a double bond, and a double bond is shorter than a single bond.
QUADRUPLE BONDS
A quadruple bond is even shorter than a triple bond, and so to make higher-order bonds, there needs to be a fairly low amount of nuclear repulsion so that the atoms can have the "flexibility" to get closer together and more easily establish an optimal equilibrium bond distance for such a high-order bond. There also needs to be fairly accessible d orbitals to accommodate for orbital occupations when making these higher-order bonds. This tends to help most when the nuclear repulsion is high, but not high enough that the d orbitals are too far away to help make these bonds occur.
(A nice balance between these two criteria facilitates high-order bonds, and this balance exists in some transition metals, such as Tungsten, Molybdenum, and Rhenium)

