What is the reaction of sodium chloride with sulfuric acid?
2 Answers
Technically, if you did want to balance it, for the sake of learning, it would be:
However, you will probably see
This equation can be written as:
It just so turns out that this is just:
...so they all cancel. So basically, no reaction happened. It's just a solvation process that doesn't actually occur as a reaction and is only something to act as a example of balancing chemical equations.
Explanation:
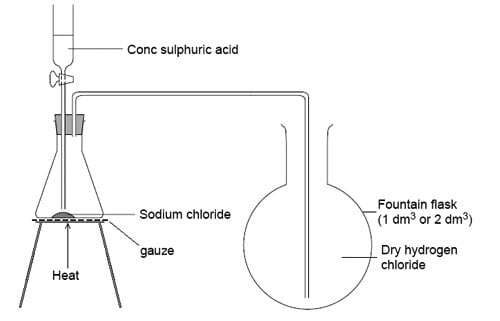
You have not provided any state information and this reaction very much depends on the reaction conditions.
If we had aqueous conditions there is no reaction.
If we have solid sodium chloride and concentrated sulphuric acid then an acid/base reaction occurs.
The sulphuric acid transfers a proton to a chloride ion:
Steamy fumes of hydrogen choride gas are observed. The other product is sodium hydrogen sulphate
This forms the basis of the laboratory preparation of hydrogen chloride gas.