Question #cbf43
1 Answer
The critical temperature (
The critical point is the temperature and pressure at which both liquid and vapor phases coexist. Above that point, the densities are pretty much the same, and the phases are indistinguishably known as a supercritical fluid (meaning "fluid above the critical point", like superposition).

If you look at the subcritical curve here, the cubic curve intersects the horizontal line three times and therefore has three solutions for the molar density (the middle being physically impossible [or spurious], A being that of the liquid, and B' being that of the gas).
At the critical point, neither phase exists more than the other, and you can see that when the three roots merge into one (labeled Pc,Tc). That is what indicates when there is only one density.
Having a boiling point implies normal conditions, where the liquid and gas are distinguishable. You can consider the boiling point inaccessible (it vanished) since neither phase is distinguishable, and there is only one observable phase. There is no enthalpy of vaporization that can convert a nonexistent liquid into a gas.
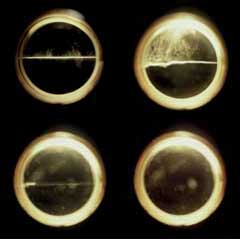
The supercritical fluid is the one where the meniscus is gone (bottom right). The separated liquid and gas is the one with a fully observable meniscus (upper left).

