How do you solve for the energy difference between #n = 1# and #n = 2# in hydrogen atom?
2 Answers
The difference in energy is solved for with the Schrodinger equation, exactly.
The actual expression is more complicated than we care about, but the energy levels for the Hydrogen atom from the first quantized energy level to the next in terms of atomic units simplifies it a lot, and is given by:
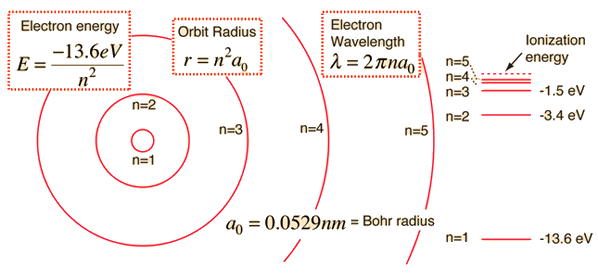
#E_n = -"13.6058 eV" cdot Z^2/n^2# where
#Z = 1# for hydrogen atom and#n# is the principal quantum number.
Hence, we take the difference (again, with
#DeltaE_(n_i->n_f) = -"13.6058 eV" cdot (1/n_f^2 - 1/n_i^2)#
So,
#color(blue)(DeltaE_(1->2)) = -"13.6058 eV" cdot (1/2^2 - 1/1^2)#
#=# #color(blue)"10.2044 eV"#
The difference is 10.2eV
You can work out the difference using the expression:
Where
(From Hyperphysics.edu)
So the difference between


