What are azeotropes according to IUPAC naming?
1 Answer
May 6, 2015
This actually isn't related to IUPAC naming. Azeotropes specifically refer to liquid-liquid solutions in which the traditional fractional distillation methods to separate two liquids by their boiling points at some point gives a composition in which both liquids are at the same richness.
Normally, you want to see this:
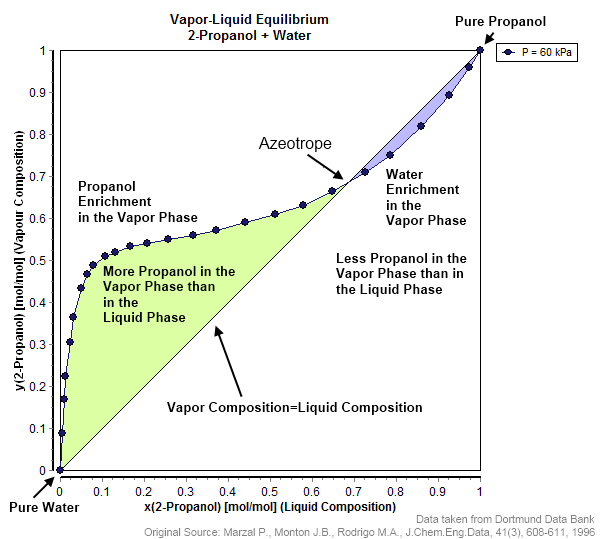
The idea is to alter the temperature of a liquid-liquid solution in a certain direction (say, down), then lower the pressure to let some liquid boil off, and repeat until a distillate remains that is solely composed of only one of the liquids.

